| NegGram® Caplets | Prescribing Information |
| (nalidixic acid, USP) | |
DESCRIPTION | CLINICAL PHARMACOLOGY | INDICATIONS AND USAGE | CONTRAINDICATIONS | WARNINGS | PRECAUTIONS | ADVERSE REACTIONS | OVERDOSAGE | DOSAGE AND ADMINISTRATION | HOW SUPPLIED | ANIMAL PHARMACOLOGY |
To reduce the development of drug-resistant bacteria and maintain the effectiveness of NegGram (nalidixic acid, USP) and other antibacterial drugs, NegGram should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
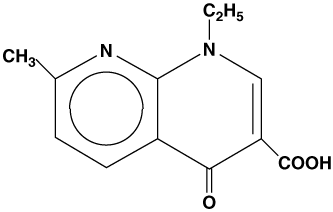
NegGram®, brand of nalidixic acid, is a quinolone antibacterial agent for oral administration. Nalidixic acid is 1-ethyl-1, 4-dihydro-7-methyl-4-oxo-1, 8-naphthyridine-3-carboxylic acid. It is a pale yellow, crystalline substance and a very weak organic acid.
Nalidixic acid has the following structural formula:
Inactive Ingredients— Hydrogenated Vegetable Oil, Methylcellulose, Microcrystalline Cellulose, Sodium Lauryl Sulfate, Yellow Ferric Oxide.
CLINICAL
PHARMACOLOGY
Following oral administration, NegGram is rapidly absorbed from the gastrointestinal
tract, partially metabolized in the liver, and rapidly excreted through
the kidneys. Unchanged nalidixic acid appears in the urine along with
an active metabolite, hydroxynalidixic acid, which has antibacterial activity
similar to that of nalidixic acid. Other metabolites include glucuronic
acid conjugates of nalidixic acid and hydroxy nalidixic acid, and the
dicarboxylic acid derivative. The hydroxy metabolite represents 30 percent
of the biologically active drug in the blood and 85 percent in the urine.
Peak serum levels of active drug average approximately 20 mcg to 40 mcg
per mL (90 percent protein bound), one to two hours after administration
of a 1 g dose to a fasting normal individual, with a half-life of about
90 minutes. Peak urine levels of active drug average approximately 150
mcg to 200 mcg per mL, three to four hours after administration, with
a half-life of about six hours. Approximately four percent of NegGram
is excreted in the feces. Traces of nalidixic acid were found in blood
and urine of an infant whose mother had received the drug during the last
trimester of pregnancy. (See PRECAUTIONS—Drug Interactions.)
Microbiology
NegGram has marked antibacterial activity against gram-negative bacteria
including Enterobacter species, Escherichia coli, Morganella
Morganii; Proteus Mirabilis, Proteus vulgaris,
and Providencia rettgeri. Pseudomonas species are generally
resistant to the drug. NegGram is bactericidal and is effective over the
entire urinary pH range. Conventional chromosomal resistance to NegGram
taken in full dosage has been reported to emerge in approximately 2 to
14 percent of patients during treatment; however, bacterial resistance
to NegGram has not been shown to be transferable via R factor.
Susceptibility Test
Diffusion Techniques: Quantitative methods that require
measurement of zone diameters give the most precise estimates of antibacterial
susceptibility. One such procedure recommended for use with a disc containing
30 mcg of nalidixic acid is the National Committee for Clinical Laboratory
Standards (NCCLS) approved procedure. Only organisms from urinary tract
infections should be tested. Results of laboratory tests using 30 mcg
nalidixic acid discs should be interpreted using the following criteria:
| Zone Diameter (mm) Interpretation | ||
| >19 | (S) Susceptible | |
| 14-18
|
(I) Intermediate | |
| <13 | (R) Resistant | |
Dilution Techniques: Broth and agar dilution methods, such as those recommended by the NCCLS, may be used to determine the minimum inhibitory concentration (MIC) of nalidixic acid. MIC test results should be interpreted according to the following criteria:
| MIC (mcg/mL) | Interpretation | |
| <16
|
(S) Susceptible | |
| >32 | (R) Resistant | |
For any susceptibility test, a report of "susceptible" indicates that the pathogen is likely to respond to nalidixic acid therapy. A report of "resistant" indicates that the pathogen is not likely to respond. A report of "intermediate" generally indicates that the test result is equivocal.
The
Quality Control strains should have the following assigned daily ranges
for nalidixic acid:
QC Strains
E. Coli
(ATCC 25922)
Disc Zone Diameter
22-28
MIC (mcg/mL)
1.0-4.0
INDICATIONS
AND USAGE
NegGram (nalidixic acid, USP) is indicated for the treatment of urinary
tract infections caused by susceptible gram-negative microorganisms, including
the majority of E. Coli, Enterobacter species, Klebsiella
species, and Proteus species. Disc susceptibility testing with
the 30 mcg disc should be performed prior to administration of the drug,
and during treatment if clinical response warrants.
To reduce the development of drug-resistant bacteria and maintain effectiveness of NegGram and other antibacterial drugs, NegGram should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered when selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
NegGram is contraindicated in patients with known hypersensitivity to
nalidixic acid or to related compounds, infants less than three months
of age, and in patients with porphyria or a history of convulsive disorders.
NegGram is contraindicated in patients undergoing concomitant therapy
with melphalan or other related cancer chemotherapeutic alkylating agents
because of serious gastrointestinal toxicity such as hemorrhagic ulcerative
colitis or intestinal necrosis.
WARNINGS
Central Nervous System (CNS) effects including convulsions, increased
intracranial pressure, and toxic psychosis have been reported with nalidixic
acid therapy. Convulsive seizures have been reported with other drugs
in this class. Quinolones may also cause CNS stimulation which may lead
to tremor, restlessness, lightheadedness, confusion, and hallucinations.
Therefore, nalidixic acid should be used with caution in patients with
known or suspected CNS disorders, such as, cerebral arteriosclerosis or
epilepsy, or other factors which predispose seizures. (See ADVERSE REACTIONS.)
If these reactions occur in patients receiving nalidixic acid, the drug
should be discontinued and appropriate measures instituted.
Serious and occasionally fatal hypersensitivity (anaphylactoid) reactions, some following the first dose, have been reported in patients receiving quinolone therapy. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, tingling, pharyngeal or facial edema, dyspnea, urticaria, and itching. Only a few patients had a history of hypersensitivity reactions. Serious anaphylactoid reactions required immediate emergency treatment with epinephrine. Oxygen, intravenous steroids, and airway management, including intubation, should be administered as indicated.
Nalidixic acid and other members of the quinolone drug class have been shown to cause arthropathy in juvenile animals. (See PRECAUTIONS and ANIMAL PHARMACOLOGY.)
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including quinolones, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of “antibiotic-associated colitis”.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis.
Peripheral neuropathy: Rare cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving quinolones, including nalidixic acid. Nalidixic acid should be discontinued if the patient experiences symptoms of neuropathy including pain, burning, tingling, numbness, and/or weakness, or is found to have deficits in light touch, pain, temperature, position sense, vibratory sensation, and/or motor strength in order to prevent the development of an irreversible condition.
Tendon Effects: Ruptures of the shoulder, hand, Achilles tendon or other tendons that required surgical repair or resulted in prolonged disability have been reported in patients receiving quinolones, including nalidixic acid. Post-marketing surveillance reports indicate that this risk may be increased in patients receiving concomitant corticosteroids, especially in the elderly. Nalidixic acid should be discontinued if the patient experiences pain, inflammation, or rupture of a tendon. Patients should rest and refrain from exercise until the diagnosis of tendonitis or tendon rupture has been excluded. Tendon rupture can occur during or after therapy with quinolones, including nalidixic acid.
PRECAUTIONS
General
Blood counts and renal and liver function tests should be performed periodically
if treatment is continued for more than two weeks. NegGram should be used
with caution in patients with liver disease, epilepsy, or severe cerebral
arteriosclerosis. (See WARNINGS.) While caution should be used in patients
with severe renal failure, therapeutic concentrations of NegGram in the
urine, without increased toxicity due to drug accumulation in the blood,
have been observed in patients on full dosage with creatinine clearances
as low as 2 mL/minute to 8 mL/minute.
Moderate to severe phototoxicity reactions have been observed in patients who are exposed to direct sunlight while receiving NegGram or other members of this drug class. Excessive sunlight should be avoided. Therapy should be discontinued if phototoxicity occurs.
If bacterial resistance to NegGram emerges during treatment, it usually does so within 48 hours, permitting rapid change to another antimicrobial. Therefore, if the clinical response is unsatisfactory or if relapse occurs, cultures and sensitivity tests should be repeated. Underdosage with NegGram during initial treatment (with less than 4 g per day for adults) may predispose to emergence of bacterial resistance. (See DOSAGE AND ADMINISTRATION.)
Cross-resistance between nalidixic acid and other quinolone derivatives such as oxolinic acid and cinoxacin has been observed.
Caution should be observed in patients with glucose-6-phosphate dehydrogenase deficiency. (See ADVERSE REACTIONS).
Prescribing NegGram in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for
Patients
Patients should be advised NegGram may be taken with or without meals.
Patients should be advised to drink fluids liberally and not take antacids.
Patients should be advised that quinolones may be associated with hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash or other allergic reactions.
Quinolones may cause dizziness and light-headedness, therefore, patients should know how they react to NegGram before they operate an automobile or machinery or engage in activities requiring mental alertness or coordination.
Patients should be advised that quinolones may increase the effects of theophylline and caffeine. There is a possibility of caffeine accumulation when products containing caffeine are consumed while taking quinolones. Patients should be advised to avoid excessive sunlight or artificial ultraviolet light while receiving nalidixic acid and to discontinue therapy if phototoxicity occurs.
Patients should be advised that convulsions have been reported in patients taking quinolones, including nalidixic acid, and to notify their physician before taking this drug if there is a history of this condition. Patients should be advised that mineral supplements, vitamins with iron or minerals, calcium-, aluminum-, magnesium-based antacids, sucralfate or Videx® , (didanosine), chewable/buffered tablets of the pediatric powder for oral solution should not be taken within the two-hour period before or within the two-hour period after taking nalidixic acid (see Drug Interactions).
Patients should be
advised:
Patients
should be counseled that antibacterial drugs including NegGram should
only be used to treat bacterial infections. They do not treat viral infections
(e.g., the common cold). When NegGram is prescribed to treat a bacterial
infection, patients should be told that although it is common to feel
better early in the course of therapy, the medication should be taken
exactly as directed. Skipping doses or not completing the full course
of therapy may (1) decrease the effectiveness of the immediate treatment
and (2) increase the likelihood that bacteria will develop resistance
and will not be treatable by NegGram or other antibacterial drugs in the
future.
Drug Interactions
Elevated plasma levels of theophylline have been reported with concomitant
quinolone use. There have been reports of theophylline-related side effects
in patients on concomitant therapy with quinolones and theophylline. Therefore,
monitoring of theophylline plasma levels should be considered and dosage
of theophylline adjusted, as required.
Quinolones have been shown to interfere with the metabolism of caffeine. This may lead to reduced clearance of caffeine and the prolongation of its plasma half-life.
Quinolones, including nalidixic acid, may enhance the effects of the oral anticoagulant warfarin or its derivatives. When these products are administered concomitantly, prothrombin time or other suitable coagulation test should be closely monitored.
Since active proliferation of organisms is a necessary condition for its antibacterial activity, the action of nalidixic acid may be inhibited by the presence of other antibacterial substances, especially bacteriostatic agents such as tetracycline, chloramphenicol, or nitrofurantoin, which is antagonistic to nalidixic acid in vitro.
Probenecid inhibits the tubular secretion of nalidixic acid and may reduce its efficacy in the treatment of urinary tract infections while increasing the risk of systemic side effects.
Serious gastrointestinal toxicity has been associated with the concomitant use of nalidixic acid and the anti-cancer drug melphalan. (see CONTRAINDICATIONS)
Antacids containing magnesium, aluminum, or calcium; sucralfate or divalent or trivalent cations such as iron; multivitamins containing zinc; and Videx®, (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution may substantially interfere with the absorption of quinolones, resulting in systemic levels considerably lower than desired. These agents should not be taken within the two-hour period before or within the two-hour period after nalidixic acid administration.
Elevated serum levels
of cyclosporine have been reported with the concomitant use of some quinolones
and cyclosporine. Therefore, cyclosporine serum levels should be monitored
and appropriate cyclosporine dosage adjustments made when these drugs
are used concomitantly.
Drug Laboratory Test Interactions
When Benedict's or Fehling's solution or Clinitest® Reagent Tablets
are used to test the urine of patients taking NegGram, a false-positive
reaction for glucose may be obtained, due to the liberation of glucuronic
acid from the metabolites excreted. However, a colorimetric test for glucose
based on an enzyme reaction (e.g., with Clinistix® Reagent Strips
or Tes-Tape® ) does not give a false-positive reaction to the liberated
glucuronic acid.
Incorrect values
may be obtained for urinary 17-keto and ketogenic steroids in patients
receiving NegGram, because of an interaction between the drug and the
m-dinitrobenzene used in the usual assay method. In such cases, the Porter-Silber
test for 17-hydroxycorticoids may be used.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In lifetime studies in the rat given nalidixic acid in the diet, there
was an increased incidence of preputial gland neoplasms in the treated
males and clitoral gland neoplasms in the treated females. Studies in
mice in which nalidixic acid was administered in the feed for two years,
or was given in the feed for 76 weeks followed by no treatment for 9 weeks,
gave equivocal evidence of carcinogenic activity.
Nalidixic acid was
tested in the Ames bacterial mutagenicity test (maximum dose 33 mcg/plate)
and the mouse lymphoma assay (L5178Y/TK; maximum dose 100 mcg/mL) with
and without metabolic activation, and results were negative.
Pregnancy: Teratogenic Effects.
Pregnancy Category C
NegGram has been shown to be teratogenic and embryocidal in rats when
given in oral doses six times the human dose. NegGram also prolonged the
duration of pregnancy especially at four times the clinical dose. There
are no adequate and well-controlled studies in pregnant women. Since nalidixic
acid, like other drugs in this class, causes arthropathy in immature animals,
NegGram should be used during pregnancy only if the potential benefit
justifies the potential risk to the fetus. (See WARNINGS and ANIMAL PHARMACOLOGY.)
Nursing Mothers
Since nalidixic acid is excreted in breast milk, it is contraindicated
during lactation.
Pediatric Use
Safety and effectiveness in infants below the age of three months have
not been established.
Usage in Patients Under 18 Years of Age
Toxicological studies have shown that nalidixic acid and related drugs
can produce erosions of the cartilage in weight-bearing joints and other
signs of arthropathy in immature animals of most species tested. No such
joint lesions have been reported in humans to date. Nevertheless, until
the significance of this finding is clarified, this drug should only be
used in patients under 18 years of age when the potential benefit justifies
the potential risk. If arthralgia occurs, treatment with nalidixic acid
should be stopped. (See WARNINGS and ANIMAL PHARMACOLOGY.)
Geriatric Use
Clinical studies of NegGram® did not include sufficient numbers of
subjects aged 65 and over to determine whether they respond differently
from younger subjects. Other reported clinical experience has not identified
differences in responses between the elderly and younger patients. Caution
should therefore be observed in using nalidixic acid in elderly patients.
This drug is known to be substantially excreted by the kidney, and the
risk of toxic reactions to this drug may be greater in patients with impaired
renal function. Because elderly patients are more likely to have decreased
renal function, care should be taken in dose selection, and it may be
useful to monitor renal function. (See PRECAUTIONS, General.)
ADVERSE
REACTIONS
Reactions reported after oral administration of NegGram include the following.
CNS effects: drowsiness, weakness, headache, dizziness and vertigo. Reversible subjective visual disturbances without objective findings have occurred infrequently (generally with each dose during the first few days of treatment). These reactions include overbrightness of lights, change in color perception, difficulty in focusing, decrease in visual acuity, and double vision. They usually disappeared promptly when dosage was reduced or therapy was discontinued. Toxic psychosis or brief convulsions have been reported rarely, usually following excessive doses. In general, the convulsions have occurred in patients with predisposing factors such as epilepsy or cerebral arteriosclerosis. In infants and children receiving therapeutic doses of NegGram, increased intracranial pressure with bulging anterior fontanel, papilledema, and headache has occasionally been observed. A few cases of 6th cranial nerve palsy have been reported. Although the mechanisms of these reactions are unknown, the signs and symptoms usually disappeared rapidly with no sequelae when treatment was discontinued.
Gastrointestinal: abdominal pain, nausea, vomiting, and diarrhea.
Allergic: rash, pruritus, urticaria, angioedema, eosinophilia, arthralgia with joint stiffness and swelling, and anaphylactoid reaction, including anaphylactic shock. Erythema Multiforme and Stevens-Johnson syndrome have been reported with nalidixic acid and other drugs in this class. Rash was the most frequently reported adverse reaction. Photosensitivity reactions consisting of erythema and bullae on exposed skin surfaces usually resolve completely in 2 weeks to 2 months after NegGram is discontinued; however, bullae may continue to appear with successive exposures to sunlight or with mild skin trauma for up to 3 months after discontinuation of drug. (See PRECAUTIONS.)
Other: rarely, cholestasis, paresthesia, metabolic acidosis, thrombocytopenia, leukopenia, or hemolytic anemia, sometimes associated with glucose 6-phosphate dehydrogenase deficiency and peripheral neuropathy.
Manifestations: Toxic psychosis, convulsions, increased intracranial pressure, or metabolic acidosis may occur in patients taking more than the recommended dosage. Vomiting, nausea, and lethargy may also occur following overdosage.
Treatment: Reactions are short-lived (two to three hours) because the drug is rapidly excreted. If absorption has occurred, increased fluid administration is advisable and supportive measures such as oxygen and means of artificial respiration should be available. Although anticonvulsant therapy has not been used in the few instances of overdosage reported, it may be indicated in a severe case.
DOSAGE
AND ADMINISTRATION
Antacids containing calcium, magnesium, or aluminum; sucralfate; divalent
or trivalent cations such as iron; multivitamins containing zinc; or Videx®
(Didanosine), chewable/buffered tablets of the pediatric powder for oral
solution should not be taken within the two-hour period before or within
the two-hour period after taking nalidixic acid.
Adults: The recommended dosage for initial therapy in adults is 1 g administered four times daily for one or two weeks (total daily dose, 4 g). For prolonged therapy, the total daily dose may be reduced to 2 g after the initial treatment period. Underdosage during initial treatment may predispose to emergence of bacterial resistance
Pediatric Patients: Until further experience is gained, NegGram should not be administered to infants younger than three months. Dosage in pediatric patients 12 years of age and under should be calculated on the basis of body weight. The recommended total daily dosage for initial therapy is 25 mg/lb/day (55 mg/kg/day), administered in four equally divided doses. For prolonged therapy, the total daily dose may be reduced to 15 mg/lb/day (33 mg/kg/day). NegGram Caplets of 250 mg may be used.
HOW
SUPPLIED
NegGram (nalidixic acid, USP) is supplied as:
Caplets of 500 mg, light buff-colored capsule-shaped tablets,
bottles of 56 (NDC 0024-1322-03)
Store at room temperature, up to 30° C (86° F).
ANIMAL
PHARMACOLOGY
NegGram (nalidixic acid) and related drugs have been shown to cause arthropathy
in juvenile animals of most species tested. (See WARNINGS.)
Long-term administration of nalidixic acid to rats resulted in retinal degeneration and cataracts.
Hydroxynalidixic acid, the principal metabolite of NegGram, did not produce any oculotoxic effects at any dosage level in seven species of animals including three primate species. However, oral administration of this metabolite in high doses has been shown to have oculotoxic potential, namely in dogs and cats where it produced retinal degeneration upon prolonged administration leading, in some cases, to blindness.
In experiments with NegGram itself, little if any such activity could be elicited in either dogs or cats. Sensitivity to CNS side effects in these species limited the doses of NegGram that could be used; this factor, together with a low conversion rate to the hydroxy metabolite in these species, may explain the absence of these effects.
![]()
Distributed
by
Sanofi-Synthelabo Inc.
New York, NY 10016
| Revised June 2004 | NSW-6 J(O) |