NASACORT® HFA
[na´ za-cort]
(triamcinolone acetonide)
Nasal Aerosol
For Intranasal Use Only
Shake Well Before Using
Rev. April 2004c
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
![]()
Triamcinolone acetonide, USP, the
active ingredient in Nasacort® HFA Nasal Aerosol,
is a glucocorticosteroid with a molecular weight of 434.5, the
chemical designation 9-Fluoro-11ß,16![]() ,17,
21-tetrahydroxypregna-1, 4-diene-3, 20-dione cyclic 16,17-acetal
with acetone (C24H31FO6), and
the following chemical structure:
,17,
21-tetrahydroxypregna-1, 4-diene-3, 20-dione cyclic 16,17-acetal
with acetone (C24H31FO6), and
the following chemical structure:
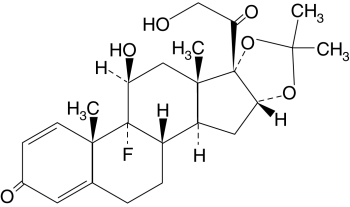
Triamcinolone acetonide is a white to cream-colored crystalline powder, practically insoluble in water, very soluble in dehydrated alcohol, chloroform, and methyl alcohol.
Nasacort HFA Nasal Aerosol is a metered-dose aerosol unit containing a microcrystalline suspension of triamcinolone acetonide in tetrafluoroethane (HFA-134a) and dehydrated alcohol USP 0.7% w/w. Each canister contains 15 mg of triamcinolone acetonide.
The canister must be primed with 3
actuations prior to the first use or after a period of non-use
(3 days). After priming, each actuation meters 100 mcg of triamcinolone
acetonide in 65 mg of suspension from the valve and delivers 55
mcg of triamcinolone acetonide from the nasal actuator to the
patient. If the product is not used for more than 3 days, it should
be re-primed with 3 actuations.
Each 9.3 g canister of Nasacort HFA Nasal Aerosol provides 100
metered sprays. After 100 metered sprays, this amount of medication
delivered per actuation may not be consistent and the unit should
be discarded. Patients are provided with a check-off card to track
usage as part of the PATIENT’S INSTRUCTIONS FOR USE
tear-off sheet.
Triamcinolone acetonide, a synthetic glucocorticosteroid, is a more potent derivative of triamcinolone. Although triamcinolone itself is approximately 1 to 2 times as potent as prednisone in animal models of inflammation, triamcinolone acetonide is approximately 8 times more potent than prednisone. The clinical relevance of in vitro or animal models of potency comparison is unknown.
The precise mechanism of corticosteroid action on allergic rhinitis is not known. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation. Nasacort HFA Nasal Aerosol, like other corticosteroids, does not have an immediate effect on allergic rhinitis signs and symptoms. When corticosteroids are discontinued, symptoms may not recur for several days.
Pharmacokinetics:
Absorption: Triamcinolone acetonide is absorbed into the systemic
circulation in humans following intranasal administration. In
a study involving 24 patients with allergic rhinitis and 24 healthy
subjects, absorption of triamcinolone from the nasal mucosa was
similar. Following a single intranasal administration of 440 mcg
of Nasacort HFA Nasal Aerosol to healthy subjects, the mean maximum
triamcinolone acetonide plasma concentration of 0.2 (SD ±
0.1) ng/mL was observed at 3.8 (SD ± 2.4) hours postdosing.
Distribution: Based on an intravenous dose of 2 mg of triamcinolone acetonide phosphate ester in 12 healthy subjects, the mean volume of distribution (Vd) was 103.4 L (SD ± 58.7). The binding of triamcinolone acetonide to plasma proteins is relatively low, and remains consistent over a wide plasma triamcinolone acetonide concentration range (0.03 - 3.2 ng/mL). Based on an ex vivo study, the overall mean percent bound to plasma protein was approximately 68% (SD ± 4.3%).
Metabolism: The metabolism and excretion of triamcinolone acetonide were both rapid and extensive with no parent compound being detected in plasma after 24 hours post oral [14C]-triamcinolone radiolabeled dose.
The disposition and metabolic profile of [14C]-triamcinolone acetonide in human plasma, urine, and feces was evaluated in 6 healthy male subjects. Three major metabolites in plasma were 6ß-hydroxytriamcinolone acetonide, triamcinolone acetonide-21-oic acid, and 6ß-hydroxytriam-cinolone acetonide-21-oic acid. Two major metabolites in the urine were 6ß-hydroxytriamcinolone acetonide and its derivative following further oxidation (possibly 6-oxo-triamcinolone acetonide or 11-oxo-triam-cinolone acetonide). A trace amount of triamcinolone acetonide-21-oic acid was also found in urine. Primary metabolites in the feces were the same as those in plasma. All three major metabolites in the plasma had no activity as determined by in vitro studies.
Elimination: Based on an intravenous dose of 2 mg of triamcinolone acetonide phosphate ester in 12 healthy subjects, the mean half-life of triamcinolone acetonide was 2 hours (SD ± 0.7), and the mean clearance was 37.3 L/hour (SD ± 12.8). Following a single intranasal administration of 440 mcg of triamcinolone acetonide in 24 healthy subjects, the mean half-life was 5.4 hours (SD ± 4.1). However, this value probably reflects lingering absorption of triamcinolone acetonide.
Following a single 800 mcg oral dose of radiolabeled [14C]-triamcinolone acetonide in 6 healthy subjects, urinary and fecal excretion accounted for approximately 90% of the oral [14C]-radiolabeled dose. Of the recovered [14C]-radioactivity, approximately 40% and 60% were found in the urine and feces, respectively. Urinary excretion of [14C]-radioactivity was essentially complete within 24 hours post-dose with most of the fecal elimination completed between 48 and 96 hours post-dose. The plasma half-life of corticosteroids does not correlate well with the duration of the drug’s activity.
Special Populations: Formal pharmacokinetic studies using intranasal triamcinolone acetonide were not carried out in any special populations. The effects of renal impairment, hepatic impairment, age, or gender on the pharmacokinetics of triamcinolone acetonide following intranasal administration have not been investigated.
Pharmacodynamics: Several studies were performed to determine if systemic absorption played a role in the response to triamcinolone acetonide in the treatment of allergic rhinitis. An open-label, multiple-dose study was conducted comparing intranasal CFC and depot intramuscular formulations of triamcinolone acetonide in 25 adult patients with seasonal or perennial allergic rhinitis. The doses used were based on bioavailability studies of each formulation. The intranasal CFC formulation was administered at a dose of 440 mcg once daily for 42 days, and the 4 mg depot intramuscular formulation was administered once a week for 42 days. Weekly injection yielded sustained plasma levels throughout the dosing interval while daily intranasal administration resulted in daily peak and trough concentrations, the mean of which was 3.5 times below the mean plasma levels achieved with intramuscular administration. Both intranasal and intramuscular triamcinolone acetonide were clinically effective on allergic rhinitis symptoms. This suggests that triamcinolone acetonide is both systemically and topically active.
The potential systemic effects of triamcinolone acetonide aqueous formulation (Nasacort AQ Nasal Spray) on the hypothalamic-pituitary-adrenal (HPA) axis were studied in 64 patients with allergic rhinitis. Nasacort AQ Nasal Spray administered to adults at doses of 220 or 440 mcg once daily was compared to placebo or 10 mg prednisone administered as oral capsules for 42 days. Plasma cortisol concentrations were not affected in patients treated with either placebo or Nasacort AQ Nasal Spray in response to a 6-hour cosyntropin stimulation test, while oral prednisone significantly reduced the response to cosyntropin.
In another trial, the potential systemic effects of triamcinolone acetonide CFC formulation (Nasacort Nasal Inhaler) on the HPA axis were studied in 64 patients with allergic rhinitis. Nasacort Nasal Inhaler administered to adults at doses of 220 or 440 mcg once daily was compared to placebo or 10 mg prednisone once daily administered as oral capsules for 42 days. Plasma cortisol concentrations, 24-hour urinary 17-OHCS, and 24-hour urinary free cortisol concentrations were not affected in patients treated with either placebo or Nasacort Nasal Inhaler in response to a 6-hour cosyntropin stimulation test, while oral prednisone significantly reduced the response to cosyntropin for plasma cortisol concentrations and 24-hour urinary 17-OHCS concentrations.
A study was conducted evaluating plasma cortisol
response 30 and 60 minutes after cosyntropin stimulation in 80
pediatric patients aged 6 to 12 years with allergic rhinitis who
received 220 mcg or 440 mcg (twice the maximum recommended daily
dose) of Nasacort AQ Nasal Spray daily for 6 weeks. No abnormal
response to cosyntropin infusion (peak serum cortisol <18 mcg/dL)
was observed after 6 weeks of dosing at 440 mcg per day.
The determination of efficacy and safety of Nasacort HFA Nasal Aerosol is based on the clinical program linking Nasacort HFA Nasal Aerosol to Nasacort Nasal Inhaler (triamcinolone acetonide CFC formulation), and by extrapolation from the known efficacy and safety of the Nasacort Nasal Inhaler. The clinical program of Nasacort HFA Nasal Aerosol included 2 studies conducted in the United States involving 1176 patients 12 to 83 years of age with allergic rhinitis, of whom 729 patients were treated with Nasacort HFA Nasal Aerosol. One study was a 2-week, double-blind, parallel-group, placebo-controlled trial comparing Nasacort HFA Nasal Aerosol to Nasacort Nasal Inhaler (triamcinolone acetonide CFC formulation) in 780 patients 18 years of age and older with seasonal allergic rhinitis. The design incorporated 2 doses of Nasacort Nasal Inhaler that were known to be effective (110 mcg and 440 mcg once daily), and 2 doses of Nasacort HFA Nasal Aerosol (110 mcg and 440 mcg once daily). Another study was a 12-month, open-label safety study in 396 patients 12 years of age and older with perennial allergic rhinitis. The dose of Nasacort HFA Nasal Aerosol was 220 mcg once daily for the first 2 weeks and 440 mcg once daily for the remainder of the study.
In the 2-week, double-blind study, Nasacort HFA Nasal Aerosol and Nasacort Nasal Inhaler (triamcinolone acetonide CFC formulation) were comparable, and both formulations showed a significant reduction in symptoms of allergic rhinitis (see table below). There were no significant differences in the effectiveness of Nasacort HFA Nasal Aerosol across subgroups of patients defined by gender, age, or race.
Effect of Nasacort HFA Nasal Aerosol on Total
Symptom Score in a
2-Week Clinical Trial in Patients with Seasonal Allergic Rhinitis.
|
Treatment Group (n)
|
Baseline Mean
Score (SEM)* |
Mean Change
From Baseline (SEM)** |
Placebo
Comparison (p-value) |
|
Nasacort HFA 440 mcg
Once Daily (111) |
6.78 (0.1)
|
-2.64 (0.18)
|
< 0.05
|
|
Nasacort HFA 110 mcg
Once Daily (105) |
6.41 (0.1)
|
-2.29 (0.18)
|
< 0.05
|
|
Placebo (109)
|
6.75 (0.1)
|
-1.39 (0.18)
|
|
| * Baseline score was an average of the morning
and evening scores of 3 symptoms of allergic rhinitis (nasal
discharge, nasal stuffiness, and sneezing) for 3 days preceding
randomization and the morning of randomization. Each symptom
was scored by patients 2 times a day by reflection over the
preceding 12 hours on a scale of 0 to 3 where 0=no symptom
and 3=severe symptoms. ** Changes were averaged over the 2-week treatment period compared to the baseline. Symptom scoring during treatment period was the same as that for the baseline. |
|||
Individualization of Dosage: Individual patients
will experience a variable time to onset and degree of symptom
relief when using Nasacort HFA Nasal Aerosol. After starting patients
on appropriate doses of Nasacort HFA Nasal Aerosol (see DOSAGE
AND ADMINISTRATION) it is recommended that the effect be assessed
in 4 to 7 days. If adequate relief has not been obtained by a
reasonable time, alternate forms of treatment should be considered.
The maximum total daily dose should not exceed 440 mcg (4 sprays
in each nostril) in patients 12 years of age and older and 220
mcg (2 sprays in each nostril) in patients 6 through 11 years
of age. There is no evidence that exceeding the recommended dose
is more effective. In general, it is always desirable to titrate
an individual patient to the minimum effective dose to reduce
the possibility of side effects. (See WARNINGS, PRECAUTIONS:
Information for Patients, and ADVERSE REACTIONS.)
Nasacort HFA Nasal Aerosol is indicated for the
treatment of the nasal symptoms of allergic rhinitis (seasonal
and perennial) in adults and children 6 years of age and older.
Safety and effectiveness of Nasacort HFA Nasal Aerosol in children
below 6 years of age have not been adequately established.
Nasacort HFA Nasal Aerosol is contraindicated in patients with a hypersensitivity to any of the ingredients.
The replacement of a systemic corticosteroid with a topical corticosteroid can be accompanied by signs of adrenal insufficiency and, in addition, some patients may experience symptoms of withdrawal, e.g., joint and/or muscular pain, lassitude, and depression. Patients previously treated for prolonged periods with systemic corticosteroids and transferred to topical corticosteroids should be carefully monitored for acute adrenal insufficiency in response to stress. In those patients who have asthma or other clinical conditions requiring long-term systemic corticosteroid treatment, too rapid a decrease in systemic corticosteroids may cause a severe exacerbation of their symptoms.
The concomitant use of intranasal corticosteroids with other inhaled corticosteroids could increase the risk of signs or symptoms of hypercorticism and/or suppression of the HPA axis.
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
Avoid spraying in eyes.
Intranasal corticosteroids may cause a reduction in growth velocity when administered to pediatric patients (see PRECAUTIONS: Pediatric Use).
Triamcinolone acetonide administered intranasally has been shown to be absorbed into the systemic circulation in humans. Patients with active rhinitis showed absorption similar to that found in normal volunteers.
Rarely, immediate hypersensitivity reactions or contact dermatitis occur after the administration of Nasacort HFA Nasal Aerosol. Rare instances of wheezing, nasal septum perforation, cataracts, glaucoma, and increased intraocular pressure have been reported following the intranasal application of corticosteroids, including triamcinolone acetonide. Because of the inhibitory effect of corticosteroids on wound healing in patients who have experienced recent nasal septal ulcers, nasal surgery or trauma, a corticosteroid should be used with caution until healing has occurred.
In clinical studies with triamcinolone acetonide administered intranasally, the development of localized infections of the nose and pharynx with Candida albicans has rarely occurred. When such an infection develops, it may require treatment with appropriate local or systemic therapy and discontinuance of treatment with Nasacort HFA Nasal Aerosol. As with any long-term topical treatment of the nasal cavity, patients using Nasacort HFA Nasal Aerosol over several months or longer should be examined periodically for evidence of Candida infection or other adverse effects on the nasal mucosa.
Intranasal corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract or in patients with untreated local or systemic fungal or bacterial infections, systemic viral or parasitic infections, or ocular herpes simplex.
When used at higher than recommended doses or in rare individuals at recommended doses, systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear. Therefore, larger than recommended doses of Nasacort HFA Nasal Aerosol should be avoided. If signs or symptoms of hypercorticism and/or suppression of HPA function occur, Nasacort HFA Nasal Aerosol should be discontinued slowly, consistent with accepted procedures for discontinuing oral steroid therapy.
Information for Patients:
Patients being treated with Nasacort HFA Nasal Aerosol should
receive the following information and instructions. This information
is intended to aid them in the safe and effective use of this
medication. It is not a disclosure of all possible adverse or
intended effects.
Patients who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles and, if exposed, to obtain medical advice.
Patients should use Nasacort HFA Nasal Aerosol at regular intervals since its effectiveness depends on regular use (see DOSAGE AND ADMINISTRATION). Individual patients will experience a variable time to onset and degree of symptom relief, and generally takes 1 week of treatment to reach maximum benefit. The patient should take the medication as directed and should not exceed the prescribed dosage. The patient should contact the physician if symptoms do not improve by a reasonable time, or if the condition worsens. Nasal irritation occurred in 6.2% of adults who used 440 mcg/day, the maximum recommended daily intranasal dose. The patient should contact the physician if nasal irritation occurs. It is advisable for patients who experience nasal septum discomfort to re-evaluate their technique in the application of Nasacort HFA Nasal Aerosol to minimize deposition of drug onto the septum.
For the proper use of this unit and to attain maximum
improvement, the patient should read and follow the accompanying
patient instructions carefully. Spraying Nasacort HFA Nasal Aerosol
directly into the eyes or onto the nasal septum should be avoided.
It is important
to shake the canister well prior to each actuation to insure that
a consistent amount is dispensed per actuation. The canister should
be discarded after 100 actuations.
Drug-Drug Interactions:
No drug interaction studies with triamcinolone acetonide have
been performed.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
In a 2-year study in rats, triamcinolone acetonide caused no treatment-related
carcinogenicity at oral doses up to 1.0 mcg/kg (approximately
1/50 of the maximum recommended daily intranasal dose in adults
and children on a mcg/m2 basis). In a 2-year study
in mice, triamcinolone acetonide caused no treatment-related carcinogenicity
at oral doses up to 3.0 mcg/kg (approximately 1/30 of the maximum
recommended daily intranasal dose in adults and children on a
mcg/m2 basis).
No evidence of mutagenicity was detected from in vitro tests (a reverse mutation test in Salmonella bacteria and a forward mutation test in Chinese hamster ovary cells) conducted with triamcinolone acetonide.
In male and female rats, triamcinolone acetonide
caused no change in pregnancy rate at oral doses up to 15 mcg/kg
(approximately 1/3 of the maximum recommended daily intranasal
dose in adults on a mcg/m2 basis). Triamcinolone acetonide
caused increased fetal resorptions
and stillbirths and decreases in pup weight and survival at doses
of 5.0 mcg/kg and above (approximately 1/10 of the maximum recommended
daily intranasal dose in adults on a mcg/m2 basis).
At 1.0 mcg/kg (approximately 1/50 of the maximum recommended daily
intranasal dose in adults on a mcg/m2 basis), it did
not induce the above mentioned effects.
Pregnancy:
Teratogenic Effects: Pregnancy category C. Triamcinolone
acetonide was teratogenic in rats, rabbits, and monkeys. In rats,
triamcinolone acetonide was teratogenic at inhalation doses of
20 mcg/kg and above (approximately 2/5 of the maximum recommended
daily intranasal dose in adults on a mcg/m2 basis).
In rabbits, triamcinolone acetonide was also teratogenic at inhalation
doses of 20 mcg/kg and above (approximately 4/5 of the maximum
recommended daily intranasal dose in adults on a mcg/m2
basis). In monkeys, triamcinolone acetonide was teratogenic at
an inhalation dose of 500 mcg/kg (approximately 20 times the maximum
recommended daily intranasal dose in adults on a mcg/m2
basis). Dose-related teratogenic effects in rats and rabbits included
cleft palate and/or internal hydrocephaly and axial skeletal defects,
whereas the effects observed in monkeys were cranial malformations.
There are no adequate and well-controlled studies in pregnant women. Therefore, Nasacort HFA Nasal Aerosol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. In addition, because there is an increase in corticosteroid production during pregnancy, most women will require a lower exogenous corticosteroid dose and many will not need corticosteroid treatment during pregnancy.
Nonteratogenic Effects: Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Such infants should be carefully monitored.
Nursing Mothers:
It is not known whether triamcinolone acetonide is excreted in
human milk. Because other corticosteroids are excreted in human
milk, caution should be exercised when this product is administered
to nursing women.
Pediatric Use:
Safety and effectiveness have not been established in pediatric
patients below the age of 6 years.
A placebo-controlled clinical growth study in children has not been conducted with Nasacort HFA Nasal Aerosol. Controlled clinical studies have shown that intranasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with intranasal corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with intranasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving intranasal corticosteroids, including Nasacort HFA Nasal Aerosol, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risks/benefits of treatment alternatives. To minimize the systemic effects of intranasal corticosteroids, including Nasacort HFA Nasal Aerosol, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
Geriatric Use:
Clinical studies of Nasacort HFA Nasal Aerosol did not include
sufficient numbers of subjects aged 65 and over to determine whether
they respond differently from younger subjects. Other reported
clinical experience has not identified differences in responses
between the elderly and younger patients.
Clinical Trials: A total of 1176 patients with allergic rhinitis were enrolled in placebo-controlled and open-label clinical studies of Nasacort HFA Nasal Aerosol.
In the placebo-controlled trial, 220 patients were treated with Nasacort HFA Nasal Aerosol for an average of 15 days (range 1-19 days). No changes in mucous membranes were noted from physical and visual examinations during this trial.
Adverse events occurring with an incidence of 3% or greater and more commonly with Nasacort HFA Nasal Aerosol arms compared to placebo irrespective of drug relationship are presented in the following table:
| Adverse Event | Nasacort HFA Nasal Aerosol 110 mcg (n=107) % |
Nasacort HFA Nasal Aerosol 440 mcg (n=113) % |
Placebo (n=111) % |
| Sneezing | 14.0 | 15.9 | 7.2 |
| Headache | 10.3 | 6.2 | 8.1 |
| Nasal irritation | 7.5 | 6.2 | 3.6 |
| Rhinitis | 4.7 | 3.5 | 1.8 |
Of the 396 patients enrolled in the 12-month open-label study, 75% received treatment for greater than 6 months. In this study, patients were treated with Nasacort HFA Nasal Aerosol at 220 mcg once daily for the first 2 weeks and 440 mcg once daily for remainder of the study. Adverse events that were considered possibly or probably related to Nasacort HFA Nasal Aerosol and reported at an incidence of 3% or greater included: headache, epistaxis, nasal septum discomfort, rhinitis, nasal burning, and sneezing.
In the open-label study only 2% of patients receiving recommended doses discontinued due to nasal adverse effects. In the rest of the patients the nasal adverse events usually did not interfere with treatment. Seven of the 18 patients who reported nasal septum discomfort had objective evidence of ulceration, abrasion, erosion, or excoriation of the nasal septum, and 22 of the 396 (5.5%) enrolled patients developed nasal septum disorders, of whom 8 had evidence of ulceration, erosion, or excoriation of the septum, and 14 had epistaxis. It is advisable for patients who experience nasal septum discomfort to re-evaluate their technique in the application of Nasacort HFA Nasal Aerosol to minimize deposition of drug onto the septum.
If recommended doses are exceeded, or if individuals taking Nasacort HFA Nasal Aerosol are particularly sensitive or take the drug in conjunction with other corticosteroids, symptoms of hypercorticism, e.g., Cushing syndrome, could occur.
In the event of accidental overdose, an increased potential for these adverse experiences may be expected, but systemic adverse experiences are unlikely (see OVERDOSAGE).
Observed During Clinical Practice: In addition to adverse events reported from clinical trials, the following events have been identified during use of Nasacort Nasal Inhaler (triamcinolone acetonide CFC formulation) in clinical practice: nasal septal perforation, infection of the nose and pharynx with Candida albicans, cataracts, glaucoma, increased intraocular pressure, wheezing, rash, pruritus, urticaria, dizziness, paresthesia, dry mouth, nausea, coughing, dyspnea, and allergic reaction. Because they were reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or possible causal connection to triamcinolone acetonide or a combination of these factors.
Recommended Doses:
Adults and Adolescents 12 years of age and older: The recommended
starting dose of Nasacort HFA Nasal Aerosol is 220 mcg per day
given as 2 sprays (55 mcg/spray) in each nostril once daily. If
needed, the dose may be increased to 440 mcg per day given as
4 sprays (55 mcg/spray) in each nostril once daily. Once the maximal
effect has been achieved, it is always desirable to titrate the
patient to the minimum effective dose.
Children 6 through 11 years of age: The recommended dose of Nasacort HFA Nasal Aerosol is 220 mcg per day given as 2 sprays (55 mcg/spray) in each nostril once daily. Once the maximal effect has been achieved, it is always desirable to titrate the patient to the minimum effective dose.
Safety and effectiveness have not been established in pediatric patients below the age of 6 years (see PRECAUTIONS, Pediatric Use).
Directions for Use:
Illustrated PATIENT’S INSTRUCTIONS FOR USE accompanies
each package.
Chronic overdosage may result in signs/symptoms of hypercorticism (see PRECAUTIONS). The acute topical application of the entire 15 mg contents of the canister may cause nasal irritation and headache. Acute overdosage with this dosage form is unlikely since one canister of Nasacort HFA Nasal Aerosol contains 15 mg of triamcinolone acetonide.
Nasacort HFA Nasal Aerosol is supplied with an aerosol canister which provides 100 metered dose actuations. The correct amount of medication delivered per actuation cannot be assured after 100 actuations have been dispensed, after which the unit should be discarded. Each actuation delivers 55 mcg triamcinolone acetonide through the nasal actuator. The Nasacort HFA Nasal Aerosol canister and accompanying nasal actuator are designed to be used together. The Nasacort HFA Nasal Aerosol canister should not be used with other nasal actuators and the supplied nasal actuator should not be used with other products’ canisters. Nasacort HFA Nasal Aerosol is supplied with a molecular sieve sachet as a propellant adsorbent and a white plastic protective cap, and enclosed in a foil laminate overwrap pouch. Patient instructions are also provided. Net weight of the canister contents is 9.3 grams.
NDC 0075-9403-43
CONTENTS UNDER PRESSURE
Avoid spraying in
eyes.
Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw container into fire or incinerator. Store at controlled room temperature, 20 to 25°C (68 to 77°F) [see USP].
Keep out of reach of children.
Rx only.
Rev. April 2004c
Manufactured for:
Aventis Pharmaceuticals Inc.
Bridgewater, NJ 08807
Manufactured by:
Aventis Pharma Ltd.
Holmes Chapel, Cheshire CW4 8BE
United Kingdom
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|