| SYNVISC ONE® | Prescribing Information |
| HYLAN G-F 20 | Rx only |
INDICATIONS FOR USE | CONTRAINDICATIONS
WARNINGS | PRECAUTIONS
POTENTIAL ADVERSE EFFECTS OF THE DEVICE ON HEALTH | PIVOTAL CLINICAL TRIAL
DETAILED DEVICE DESCRIPTION | HOW SUPPLIED
DIRECTIONS FOR USE | SYNVISC ONE® HYLAN G-F 20 PATIENT INFORMATION
Package contents provided sterile.
Genzyme Biosurgery, a division of Genzyme Corporation
1125 Pleasant View Terrace
Ridgefield, New Jersey 07657
Telephone: 1-888-3-SYNVISC (1-888-379-6847)
www.synvisc.com
BACK TO TOP
Information for Prescribers
Caution: Federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner).
BACK TO TOP
DESCRIPTION
Synvisc-One® (hylan G-F 20) is an elastoviscous high molecular weight fluid containing hylan A and hylan B polymers produced from chicken combs. Hylans are derivatives of hyaluronan (sodium hyaluronate). Hylan G-F 20 is unique in that the hyaluronan is chemically crosslinked. Hyaluronan is a long-chain polymer containing repeating disaccharide units of Na-glucuronate-N-acetylglucosamine.
BACK TO TOP
INDICATIONS FOR USE
Synvisc-One is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative nonpharmacologic therapy and simple analgesics, e.g., acetaminophen.
BACK TO TOP
CONTRAINDICATIONS
- Do not administer to patients with known hypersensitivity (allergy) to hyaluronan (sodium hyaluronate) preparations.
- Do not inject Synvisc-One in the knees of patients having knee joint infections or skin diseases or infections in the area of the injection site.
BACK TO TOP
WARNINGS
- Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence.
- Do not inject Synvisc-One extra-articularly or into the synovial tissues and capsule.
- Intravascular injections of Synvisc-One may cause systemic adverse events.
BACK TO TOP
PRECAUTIONS
General
- The safety and efficacy of Synvisc-One in locations other than the knee and for conditions other than osteoarthritis have not been established.
- The safety and effectiveness of the use of Synvisc-One concomitantly with other intra-articular injectables have not been established.
- Use caution when injecting Synvisc-One into patients who are allergic to avian proteins, feathers or egg products.
- The safety and efficacy of Synvisc-One in severely inflamed knee joints have not been established.
- Strict aseptic administration technique must be followed.
- STERILE CONTENTS. The syringe is intended for single use. The contents of the syringe must be used immediately after its packaging is opened. Discard any unused Synvisc-One.
- Do not use Synvisc-One if package is opened or damaged. Store in original packaging (protected from light) at room temperature below 86°F (30°C). DO NOT FREEZE.
- Remove any synovial fluid or effusion before injecting Synvisc-One.
- Synvisc-One should be used with caution when there is evidence of lymphatic or venous stasis in the leg to be injected.
Information for Patients
- Provide patients with a copy of the Patient Labeling prior to use.
- Mild to moderate pain, swelling and/or effusion of the injected knee have been reported in clinical trials that were related to intra-articular injection of Synvisc-One. These events were typically transient and usually resolved on their own or with conservative treatment.
- As with any invasive joint procedure, it is recommended that the patient avoid strenuous activities (for example, high-impact sports such as soccer, tennis or jogging) or prolonged weight-bearing activities for approximately 48 hours following the intra-articular injection. The patient should consult his or her physician regarding the appropriate time to resume such activities.
Use in Specific Populations
- Pregnancy: The safety and effectiveness of Synvisc-One have not been established in pregnant women.
- Nursing mothers: It is not known if Synvisc-One is excreted in human milk. The safety and effectiveness of Synvisc-One have not been established in lactating women.
- Pediatrics: The safety and effectiveness of Synvisc-One have not been established in pediatric patients. Pediatric patients are defined as patients ≤ 21 years of age.
BACK TO TOP
POTENTIAL ADVERSE EFFECTS OF THE DEVICE ON HEALTH
Reported Device-Related Adverse Events
The most commonly reported adverse events associated with Synvisc-One are the following:
- Arthralgia
- Arthritis
- Arthropathy
- Injection site pain
- Joint effusion
A complete list of the frequency and rate of adverse events identified in the clinical study are provided in the Safety section (Table 3).
Potential Adverse Events
The following adverse events are among those that may occur in association with intra-articular injections, including Synvisc-One
- Arthralgia
- Joint stiffness
- Joint effusion
- Joint swelling
- Joint warmth
- Injection site pain
- Arthritis
- Arthropathy
- Gait disturbance
A complete list of the frequency and rate of adverse events identified in the clinical study are provided in the Safety section (Table 2).
Post-marketing Experience
SYNVISC® (3-injection regimen) post-marketing experience has identified the following systemic events to occur rarely with administration: rash, hives, itching, fever, nausea, headache, dizziness, chills, muscle cramps, paresthesia, peripheral edema, malaise, respiratory difficulties, flushing and facial swelling. There have been rare reports of thrombocytopenia coincident with SYNVISC (3-injection regimen) injection.
Hypersensitivity reactions including anaphylactic reaction, anaphylactoid reaction, anaphylactic shock and angioedema have been reported.
BACK TO TOP
PIVOTAL CLINICAL TRIAL
Study Design
To determine the safety and effectiveness of a single injection regimen of Synvisc-One in the reduction of the pain score in osteoarthritis of the knee, a prospective, randomized, double-blind, 2-arm (parallel group) clinical trial in 21 centers in six European countries was conducted. A total of 253 patients were randomly assigned to study treatment; 123 received 6 mL of Synvisc-One and 130 received 6 mL of Phosphate-Buffered Saline. Neither the patients nor the clinical observers knew the patients' treatment allocations. The outcome measures collected included the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC; Likert 3.1 A version); patient global assessment (PTGA); clinical observer global assessment (COGA); and use of rescue analgesic (see Treatment and Evaluation Schedule). The intent-to-treat (ITT) population (all patients randomized) was used for the primary analysis. The primary efficacy analysis was a comparison over 26 weeks between the two treatment groups of change from baseline in the WOMAC A (Pain) Subscale (see Patient Population and Demographics), performed by analysis of covariance (ANCOVA).
Patient Population and Demographics
Study patients had primary osteoarthritis of the knee per American College of Rheumatology criteria and were at least 40 years old. The diagnosis was confirmed via recent radiograph showing at least one osteophyte in the target knee. Study patients had continued target knee pain despite use of conservative treatment and analgesics/non-steroidal anti-inflammatory drugs (NSAIDs). Patients with severe disease (Grade IV) per Kellgren-Lawrence criteria, or who had prior arthroplasty in the target knee, were excluded. At the beginning of the study, subjects had moderate or severe target knee pain when walking on a flat surface (on a 5-point Likert scale where 0 = none, 1 = mild, 2 = moderate, 3 = severe 4 = extreme), and an average score of 1.5 to 3.5 on the five questions of the WOMAC A (Pain) Subscale. The WOMAC A Subscale asks study subjects to rate their degree of pain when:
- Walking on a flat surface
- Going up and down stairs
- Resting during the night
- Sitting or lying
- Standing upright
Table 1 summarizes the demographics and baseline characteristics. There were no clinically meaningful differences between treatment groups in any baseline parameter.
Treatment and Evaluation Schedule
Initial Treatment Phase
Patients were followed for 26 weeks. Study visits were scheduled for screening, baseline, and weeks 1, 4, 8, 12, 18 and 26. Injections were performed aseptically at the baseline visit after arthrocentesis to withdraw any effusion or synovial fluid present. Patients were not permitted to take long-acting NSAIDs (including cyclo-oxygenase II inhibitors), opioid analgesics or corticosteroids (by any route) during the study, but were permitted to take up to 4 g per day of acetaminophen as needed for "rescue" of injected knee pain. "Rescue" medication was not permitted within 48 hours of any study visit. Injected knee assessment, patient and clinician global assessments (PTGA & COGA), WOMAC and safety evaluations were performed at each study visit.
Repeat Treatment Phase
If patients in either blinded treatment group had at least mild pain in the injected knee at the week 26 visit (and did not experience any significant clinical concerns after the first treatment administration), they were offered an injection of (open-label) Synvisc-One. Those who chose to receive the second injection were followed for 4 weeks for safety only.
Adverse Event Summary
The frequency and type of adverse events (AEs) were similar between the group of patients that received Synvisc-One and the group that received saline control.
Initial Treatment Phase: The overall proportions of patients with Treatment-Emergent AEs regardless of device relatedness (Synvisc-One: n=70, 56.9%; Saline Control: n=79, 60.8%) and with injected knee AEs regardless of device relatedness (Synvisc-One: n=44, 35.8 %; Saline Control: n=44, 33.8 %) were comparable between the two treatment groups (See Table 2). Table 3 lists the incidences of AEs in the injected knee that were assessed by the investigator to be device-related, defined as related to either the study injection or the study treatment.
Device-related AEs involving the injected knee were mild or moderate in nature and were treated symptomatically. There were no serious AEs in the injected knee in either the Synvisc-One or the saline control group.
Repeat Treatment Phase: The repeat treatment phase evaluated the safety profile of the initial phase of patients receiving a second injection of Synvisc-One. One hundred and sixty patients were treated during this phase of the study, of which 77 patients received a second injection of Synvisc-One. Of these 77 patients, 4 (5.2%) experienced five device related AEs in the injected knee. All such events were mild to moderate and were treated symptomatically. These events were arthralgia (n=2), arthritis (n=1), injection hematoma (n=1) and injection site pain (n=1). Patients who developed injected knee AEs during the initial phase of the study, and who subsequently received repeat treatment, did not experience injected knee AEs upon repeat exposure to Synvisc-One.
Overall Injected Knee Safety Summary: The safety profile of Synvisc-One is similar to the Clinical and Post-marketing experience seen with SYNVISC (3 injection regimen) where pain, swelling and effusion were the most frequently occurring AEs in the injected knee.
Cases of acute inflammation, characterized by joint pain, swelling, effusion and sometimes joint warmth and/or stiffness, have been reported following an intra-articular injection of Synvisc-One. Analysis of synovial fluid reveals aseptic fluid with no crystals. This reaction often responds within a few days to treatment with Non Steroidal Anti Inflammatory Drugs (NSAIDs), intra-articular steroids and/or arthrocentesis.
Clinical benefit from the treatment may still be apparent after such reactions.
Adverse Events Outside of the Injected Knee
Overall 101 patients (Synvisc-One: n=47, 38.2%; Saline Control: n=54, 41.5%) experienced at least one AE outside the injected knee regardless of device relatedness. The most commonly occurring (5 % or greater in either group) AEs outside the injected knee were headache, back pain, nasopharyngitis and influenza. In the Synvisc-One group there was one AE of syncope considered device-related.
No new systemic AEs were identified during this study as compared to SYNVISC.
Primary Efficacy Endpoint:
The primary endpoint for the study, the difference between the treatment groups in change from baseline over 26 Weeks in the WOMAC A Pain Score (Table 4) was met.
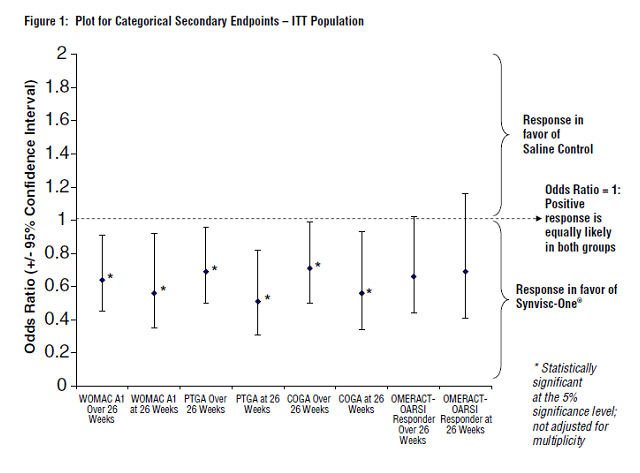
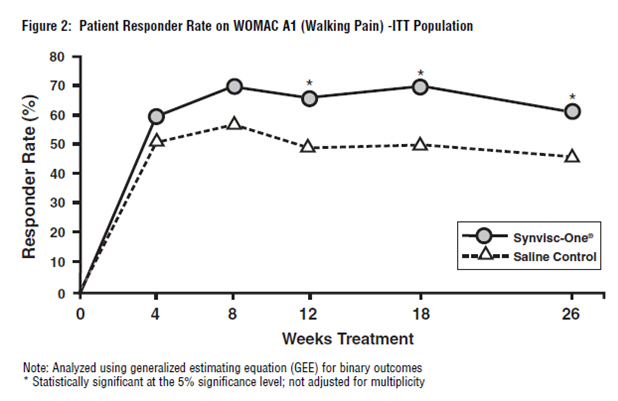
Synvisc-One also demonstrated superiority to saline control in multiple pre-defined secondary outcome measures, which included PTGA over and at 26 weeks, COGA over and at 26 weeks, and pain while walking on a flat surface (WOMAC A1) over and at 26 weeks (see Figure 1 and Table 5).
The WOMAC A1 responder rate (where response was defined as a 1-or-more category improvement from baseline and the patient did not withdraw from the study) was significantly higher in the Synvisc-One group than in the saline control group. Seventy-one percent (71%) of the patients were responders at week 18 in the Synvisc-One group (versus 54% in the saline control group). At week 26, 64% of patients in the Synvisc-One group were responders, while only 50% of patients in the saline control group were responders.
BACK TO TOP
DETAILED DEVICE DESCRIPTION
Synvisc-One combines the three doses of SYNVISC (hylan G-F 20) which consists of hylan A (average molecular weight 6,000,000 daltons) and hylan B hydrated gel in a buffered physiological sodium chloride solution, pH 7.2. Synvisc-One has an elasticity (storage modulus G') at 2.5 Hz of 111 ± 13 Pascals (Pa) and a viscosity (loss modulus G") of 25 ± 2 Pa (elasticity and viscosity of knee synovial fluid of 18 to 27- year-old humans measured with a comparable method at 2.5 Hz: G' = 117 ± 13 Pa; G" = 45 ± 8 Pa.)
Each 10 mL syringe of Synvisc-One combines the three 2-mL doses (16 mg each) of a complete SYNVISC treatment regimen (48 mg). Each Synvisc-One 10-mL syringe contains:
- Hylan polymers (hylan A + hylan B) 48 mg
- Sodium chloride 51 mg
- Disodium hydrogen phosphate 0.96 mg
- Sodium dihydrogen phosphate monohydrate 0.24 mg
- Water for injection q.s. to 6.0 mL
BACK TO TOP
HOW SUPPLIED
Synvisc-One is supplied in a 10 mL glass syringe containing 3 doses (48 mg) of hylan G-F 20. The contents of the syringe are sterile and non-pyrogenic.
BACK TO TOP
DIRECTIONS FOR USE
Precaution: Do not use Synvisc-One if the package has been opened or damaged. Store in the original packaging (protected from light) at room temperature below 86°F (30°C). DO NOT FREEZE.
Precaution: The syringe containing Synvisc-One is intended for single use. The contents of the syringe must be used immediately after the syringe has been removed from its packaging.
Precaution: Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence.
Synvisc-One is administered as a single intra-articular. Strict aseptic administration technique must be followed.
- Using an 18- to 20-gauge needle, remove synovial fluid or effusion before injecting Synvisc-One.
- Do not use the same syringe for removing synovial fluid and for injecting Synvisc-One; however the same 18- to 20-gauge needle should be used.
- Twist the tip cap before pulling it off, as this will minimize product leakage.
- To ensure a tight seal and prevent leakage during administration, secure the needle tightly while firmly holding the luer hub.
Precaution: Do not over tighten or apply excessive leverage when attaching the needle or removing the needle guard, as this may break the syringe tip.
- Inject the full 6 mL in one knee only.
BACK TO TOP
MANUFACTURED AND DISTRIBUTED BY:
Genzyme Biosurgery a division of Genzyme Corporation
1125 Pleasant View Terrace
Ridgefield, New Jersey 07657
Telephone: 1-888-3-SYNVISC (1-888-379-6847)
SYNVISC-ONE, SYNVISC and GENZYME are registered trademarks of Genzyme Corporation.
BACK TO TOP
SYNVISC ONE®
HYLAN G-F 20
PATIENT INFORMATION
Be sure to read the following important information carefully. This information does not take the place of your doctor's advice. If you do not understand this information or want to know more, ask your doctor.
Glossary of Terms
Hyaluronan (pronounced hy-al-u-ROE-nan): is a natural substance that is present in very high amounts in joints. It acts like a lubricant and a shock absorber in the joint and is needed for the joint to work properly.
Non-steroidal anti-inflammatory drugs: also known as "NSAIDs"; medication used to treat pain or swelling. There are many examples of NSAIDs, including (but not limited to) aspirin and ibuprofen. Some of these are over-the-counter drugs, and some can only be obtained by prescription.
Osteoarthritis (pronounced OS-te-o-arth-RI-tis): (OA) is a type of arthritis that involves the wearing down of cartilage (the protective covering on the ends of your bones) and loss of cushioning fluid in the joint
Table of Contents
- -Glossary of Terms
- -Table of Contents
- -What is the Synvisc-One® product?
- -How is the Synvisc-One® product used? (Indications)
- -How is the Synvisc-One® product given?
- -Are there any reasons why I should not receive a Synvisc-One® injection? (Contraindications)
- -What should my doctor warn me about?
- -What are the risks of getting a Synvisc-One® injection?
- -What are the benefits of getting a Synvisc-One® injection?
- -What do I need to do after I get Synvisc-One® injection?
- -What other treatments are available for OA?
- - Non-drug treatments
- - Drug therapy
- -When should I call my doctor? (Troubleshooting)
- -What adverse events were observed in the clinical study?
- -How do I get more information about the Synvisc-One® product? (User Assistance)
What is the Synvisc-One® product?
Synvisc-One is a gel-like mixture that comes in a syringe containing 6 mL (1 ½ teaspoon) and is injected into your knee. It is made up of hylan A fluid, hylan B gel, and salt water. Hylan A and hylan B are made from a substance called hyaluronan (pronounced hy-al-u-ROE-nan), also known as sodium hyaluronate that comes from chicken combs. Hyaluronan is a natural substance found in the body and is present in very high amounts in joints. The body's own hyaluronan acts like a lubricant and a shock absorber in the joint and is needed for the joint to work properly.
How is the Synvisc-One® product used? (Indications)
The FDA-approved indication for Synvisc-One is:
Synvisc-One is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy and simple analgesics, e.g., acetaminophen.
How is the Synvisc-One® product given?
Your doctor will inject Synvisc-One into your knee.
Are there any reasons why I should not receive a Synvisc-One® injection? (Contraindications)
Your doctor will determine if there is any reason why you are not an appropriate candidate for Synvisc-One. You should be aware that Synvisc-One:
- Should not be used in patients who have had any prior allergic reactions to SYNVISC, Synvisc-One or any hyaluronan-based products. Signs of an allergic reaction may include swelling of your face, tongue, or throat; difficulty breathing or swallowing; shortness of breath; wheezing; chest pain; a tightness in your throat; sleepiness; rash; itching; hives; flushing; and/or fever.
- Should not be used in patients with a knee joint infection, skin disease or infection around the area where the injection will be given.
What should my doctor warn me about?
The following are important treatment considerations for you to discuss with your doctor and understand in order to help avoid unsatisfactory results and complications:
- Synvisc-One is only for injection into the knee, performed by a doctor or other qualified health care professional. Synvisc-One has not been tested to show pain relief in joints other than the knee.
- Synvisc-One has not been tested to show better pain relief when combined with other injected medicines.
- Tell your doctor if you are allergic to products from birds such as feathers, eggs, and poultry.
- Tell your doctor if you have significant swelling or blood clots in the leg.
- Synvisc should be used with caution when there is evidence of lymphatic or venous stasis in the leg to be injected.
- Synvisc-One has not been tested in pregnant women, or women who are nursing. You should tell your doctor if you think you are pregnant, or if you are nursing a child.
- Synvisc-One has not been tested in children (≤ 21 years of age).
What are the risks of getting a Synvisc-One® injection?
The side effects (also called reactions) sometimes seen after any injection into the knee, including Synvisc-One, include: pain, swelling, heat, redness, and/or fluid build-up around the knee. These reactions are generally mild and do not last long. Reactions are generally treated by resting and applying ice to the injected knee. Sometimes it is necessary to give pain relievers by mouth such as acetaminophen or NSAIDs, or to give injections of steroids, or to remove fluid from the knee joint. Patients rarely undergo arthroscopy (a surgical inspection of the knee joint) or other medical procedures related to these reactions.
Other side effects seen with SYNVISC or Synvisc-One are: rashes, hives, itching, muscle pain/cramps, flushing and/or swelling of your face, fast heart beat, nausea (or feeling sick to your stomach), dizziness, fever, chills, headache, difficulty breathing, swelling in your arms and/or legs, prickly feeling of your skin, and in rare cases a low number of platelets in the blood (platelets are a type of blood cell that are needed to help your blood clot when you are cut or injured). Allergic reactions, some which can be potentially severe, were observed during the use of Synvisc-One.
Rare cases of knee joint infection have been reported after SYNVISC injections. If any of the above side effects or symptoms appear after you are given Synvisc-One, or if you have any other problems, you should call your doctor.
What are the benefits of getting a Synvisc-One® injection?
As shown in a medical study of 253 patients with osteoarthritis (OA) of the knee, where approximately half received either a single injection of Synvisc-One or an injection of the same volume of salt water (a "Saline Control" injection), the major benefits of Synvisc-One are pain relief and improvement in other symptoms related to OA of the knee.
What do I need to do after I get Synvisc-One® injection?
It is recommended you avoid strenuous activities (for example, high-impact sports such as tennis or jogging) or prolonged weight-bearing activities for approximately 48 hours following the injection. You should consult your doctor regarding the appropriate time to resume such activities.
What other treatments are available for OA?
If you have OA, there are other things you can do besides getting Synvisc-One. These include:
Non-drug treatments
- Avoiding activities that cause knee pain
- Exercise or physical therapy
- Weight loss
- Removal of excess fluid from your knee
Drug therapy
- Pain relievers such as acetaminophen and narcotics
- Drugs that reduce inflammation (signs of inflammation are swelling, pain or redness), such as aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs, for example ibuprofen and naproxen)
- Steroids that are injected directly into your knee.
When should I call my doctor? (Troubleshooting)
If any of the side effects or symptoms described above appear after you are given Synvisc-One, or if you have any other problems, you should call your doctor.
What did the clinical studies show?
A study was conducted in 6 countries outside the United States with 21 physicians. The patients in the study had mild to moderate knee OA, moderate to severe pain, and did not have sufficient relief of their pain and symptoms with medications taken by mouth.
A total of 253 patients in the study were assigned by chance to receive either a single injection of Synvisc-One (n=123 patients), or an injection of the same volume of salt water (a "Saline Control" injection) (n=130 patients). Neither the patients nor the doctors evaluating them knew which treatment they received. Any fluid that was present in the patient's knee was removed before the injection. The patients were seen by their doctor at standard times over 6 months. Information was collected about how much pain they were experiencing doing various types of activities, how much they were limited in their daily activities by their OA, and on their overall condition. Their doctor also provided an overall rating of their OA.
The main measure of the study was how much pain the subjects had doing five common types of activities over the 6 months duration of the study. Daily activity limitations and overall evaluations were also compared between the group of patients receiving Synvisc-One injection and the group receiving salt water injection. The study showed that patients receiving Synvisc-One had significantly less pain over 6 months, and felt significantly better than the patients who received the salt water injections. The difference in pain score reduction from baseline to 6 months between the Synvisc-One and salt water control injection was 0.15 out of a 5 point scale for the measurement of OA pain in the knee.
What adverse events were observed in the clinical study?
The following are the most common adverse events that occurred during the clinical trial of Synvisc-One:
- Pain in the knee or at the injection site
- Stiffness, swelling or warmth in or around the knee
- Changes in the way that you walk (e.g., limping)
Severe adverse events were not observed in the Synvisc-One trial. Joint infections did not occur in the injected knee in the Synvisc-One clinical trial. The most commonly occurring adverse events outside of the injected knee were headache, back pain, sore throat and the flu. One patient had a single episode of feeling faint.
How do I get more information about the Synvisc-One® product? (User Assistance)
If you have any questions or would like to find out more about Synvisc-One, you may call Genzyme Biosurgery at 1-888-3-SYNVISC (1-888-379-6847) or visit www.synvisc.com.
MANUFACTURED AND DISTRIBUTED BY:
Genzyme Biosurgery
A division of Genzyme Corporation
1125 Pleasant View Terrace
Ridgefield, New Jersey 07657
SYNVISC-ONE, SYNVISC and GENZYME are registered trademarks of Genzyme Corporation.
BACK TO TOP
| Parameter/Category | Synvisc-One® (N=124)* |
Saline Control (N=129)* |
Total (N=253) |
|---|---|---|---|
| Age, n * | 124 | 129 | 253 |
| Mean (SD) | 63.6 (9.6) | 62.5 (9.2) | 63.0 (9.4) |
| Range | 42, 83 | 43, 84 | 42, 84 |
| Sex, n * | 124 | 129 | 253 |
| Female, n (%) | 92 (74%) | 88 (68%) | 180 (71%) |
| Race, n * | 124 | 129 | 253 |
| Caucasian, n (%) | 118 (95%) | 125 (97%) | 243 (96%) |
| Non-Caucasian, n (%) | 6 (5%) | 4 (3%) | 10 (4%) |
| Body Mass Index (kg/m²), n* | 123 | 129 | 252 |
| Mean (SD) | 29.1 (4.8) | 29.8 (5.7) | 29.4 (5.4) |
| Range | 20.7, 46.0 | 19.5, 52.4 | 19.5, 52.4 |
| Prior Corticosteroids In Target Knee, n† | 123 | 130 | 253 |
| Yes – n (%) | 40 (32%) | 31 (24%) | 71 (28%) |
| Prior Arthroscopy In Target Knee, n † | 123 | 130 | 253 |
| Yes – n (%) | 26 (21%) | 28 (22%) | 54 (21%) |
| Tibio-Femoral Joint Modified Kellgren-Lawrence Numerical Grading System† | |||
| Grade II | 63 (51%) | 51 (39%) | 114 (45%) |
| Grade III | 60 (49%) | 78 (60%) | 138 (55%) |
| Grade IV | 0 | 1 (1%) | 1 (0%) |
| Total WOMAC Score (0–96); Mean (SD) * | 55.1 (10.5) | 54.8 (9.4) | |
| WOMAC A Score (0–4); Mean (SD) * | 2.30 (0.43) | 2.25 (0.41) | |
| PTGA – Mean (SD) (0–4) * | 2.57 (0.67) | 2.50 (0.64) | |
| COGA – Mean (SD) (0–4) * | 2.44 (0.76) | 2.49 (0.75) | |
| MedDRA Preferred Term | Synvisc-One® N=123 n (%) |
Saline Control N=130 n (%) |
|---|---|---|
| Note: Patients are counted once for each unique AE regardless of device relatedness, and may have had more than one unique AE. | ||
| Any Treatment-Emergent Adverse Event | 44 (35.8%) | 44 (33.8%) |
| Arthralgia | 31 (25.2%) | 28 (21.5%) |
| Joint stiffness | 10 (8.1%) | 13 (10.0%) |
| Joint effusion | 7 (5.7%) | 7 (5.4%) |
| Joint swelling | 5 (4.1%) | 7 (5.4%) |
| Joint warmth | 2 (1.6%) | 5 (3.8%) |
| Post-traumatic pain | 0 | 3 (2.3%) |
| Injection site pain | 1 (0.8%) | 1 (0.8%) |
| Synovial cyst | 0 | 2 (1.5%) |
| Arthritis | 1 (0.8%) | 0 |
| Arthropathy | 1 (0.8%) | 0 |
| Gait disturbance | 1 (0.8%) | 0 |
| Joint range of motion decreased | 0 | 1 (0.8%) |
| Ostheoarthritis | 0 | 1 (0.8%) |
| MedDRA Preferred Term | Synvisc-One® N=123 n (%) |
Saline Control N=130 n (%) |
|---|---|---|
| Note: Patients are counted once for each unique AE, and may have had more than one unique AE. | ||
| Any Device-Related Adverse Event | 7 (5.7%) | 4 (3.1%) |
| Arthralgia | 2 (1.6%) | 3 (2.3%) |
| Arthritis | 1 (0.8%) | 0 |
| Arthropathy | 1 (0.8%) | 0 |
| Injection site pain | 1 (0.8%) | 1 (0.8%) |
| Joint effusion | 2 (1.6%) | 0 |
| Baseline Mean (SE) (0–4 Scale) |
Mean Post-treatment (SE) (0–4 Scale) |
Estimated Change (SE) | Estimated Difference from Saline Contol (95% CI) | p-value (ANCOVA) | |
|---|---|---|---|---|---|
| WOMAC A scale using 5 point Likert scale, where 0 = no pain and 4 = extreme pain | |||||
| Repeated measures Analysis of Covariance was used for the WOMAC A pain score change from the baseline. | |||||
| Synvisc-One® (n=124) |
2.30 (0.04) |
1.43 (0.06) |
-0.84 (0.06) |
0.15 (-0.302, -0.002) |
0.047 |
| Saline Control (n=129) |
2.25 (0.04) |
1.59 (0.06) |
-0.69 (0.06) |
||
| Odds Ratio* | Definition | Explanation | ||
|---|---|---|---|---|
| Generalized Estimating Equation for categorical data | ||||
| Odds ratio = (Probability [Worse] / Probability [Better]) for Synvisc-One / Probability [Worse] / Probability [Better]) for Control If odds ratio <1, then in favor of Synvisc-One | ||||
| WOMAC A1 | Over 26 weeks |
0.64† | The odds (probability [Worse] / Probability [Better]) for Synvisc-One for over 26 weeks and at 26 weeks is approximately 64%, and 56%, respectively, to the odds for control. | Synvisc-One patients were 1.56 times more likely to self-report pain relief while walking on a flat surface compared to those patients treated with saline control over 26 weeks and 1.79 times more likely to self-report pain relief while walking on a flat surface compared to those patients treated with saline control at 26 weeks. |
| At week 26 | 0.56† | |||
PTGA |
Over 26 weeks | 0.69† | The odds (probability [Worse] / Probability [Better]) for Synvisc-One for over 26 weeks and at 26 weeks is approximately 69% and 51%, respectively, to the odds for control. PTGA: Patient Global Assessment has 5 scales (Very well, Well, Fair, Poor, Very poor) |
Synvisc-One patients were 1.45 times more likely to self-report improvement in overall health status compared to those patients treated with saline control over 26 weeks and 1.96 times more likely to self-report improvement in overall health status compared to those patients treated with saline control at 26 weeks. |
| At week 26 | 0.51† | |||
COGA |
Over 26 weeks | 0.71† | The odds (probability [Worse] / Probability [Better]) for Synvisc-One for over 26 weeks and at 26 weeks is approximately 71%, and 56%, respectively, to the odds for control. COGA: Clinical Observer Global Assessment has 5 scales (Very well, Well, Fair, Poor, Very poor) |
Blinded clinical observers were 1.41 times more likely to assess patients treated with Synvisc-One as showing overall improvement in disease status compared to those patients treated with saline control over 26 weeks and 1.79 times more likely to assess patients treated with Synvisc-One as showing overall improvement in disease status compared to those patients treated with saline control at 26 weeks. |
| At week 26 | 0.56† | |||
| OMERACT-OARSI Responder | Over 26 weeks | 0.66 | This response analysis did not reach statistical significance between, the treatment groups. | |
| At week 26 | 0.69 | |||
| Estimate of Treatment Difference (Analysis of Covariance) |
||||
| WOMAC C | Over 26 weeks | -0.18 | The study did not show a statistically significant difference in functional improvement between the treatment groups. | |
| At week 26 | -0.11 | |||
Revised Sep 2014
HYL-1-WFSPLW-WSPLW-SEP14