| PEDIACOF® |
Prescribing Information |
DESCRIPTION |
CLINICAL PHARMACOLOGY
|
INDICATIONS
AND USAGE | CONTRAINDICATIONS
WARNINGS |
PRECAUTIONS
ADVERSE
REACTIONS |
OVERDOSAGE
DOSAGE
AND ADMINISTRATION |
HOW SUPPLIED
DESCRIPTION
Each teaspoon (5 mL) contains:
| Codeine phosphate, USP................................... | 5.0 mg |
| (Warning: May be habit forming.) | |
| Phenylephrine hydrochloride, USP..................... | 2.5 mg |
| Chlorpheniramine maleate, USP........................ | 0.75 mg |
| Potassium iodide, USP..................................... | 75.0 mg |
with sodium benzoate 0.2% as preservative and alcohol 5%.
PEDIACOF is a pleasant-tasting,
raspberry-flavored cough syrup. It contains four active ingredients in
proper proportion for children. Codeine phosphate is a white crystalline,
odorless powder which is freely soluble in water. It is a narcotic analgesic.
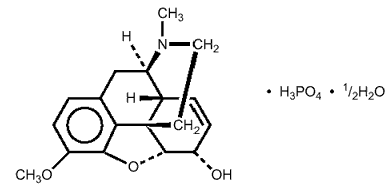
Codeine phosphate is 7,8-Didehydro-4,5 -epoxy-3-methoxy-17-methylmorphinan-6
-epoxy-3-methoxy-17-methylmorphinan-6 -ol
phosphate, and has the following structural formula:
-ol
phosphate, and has the following structural formula:
Phenylephrine hydrochloride is a vasoconstrictor and pressor drug chemically
related to epinephrine and ephedrine. It is a synthetic sympathomimetic
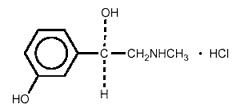
agent. Chemically, phenylephrine hydrochloride is (—)-m-Hydroxy- -[(methylamino)
methyl]-benzyl alcohol hydrochloride, and has the following structural
formula:
-[(methylamino)
methyl]-benzyl alcohol hydrochloride, and has the following structural
formula:
Chlorpheniramine maleate is an alkylamine H1-blocking agent (antihistamine)
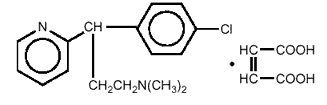
which is chemically 2-Pyridinepropanamine,  -(4-chlorphenyl)- N,N-dimethyl-,(Z)
- 2-butenedioate with the following structural formula:
-(4-chlorphenyl)- N,N-dimethyl-,(Z)
- 2-butenedioate with the following structural formula:
Potassium iodide is an expectorant.
Inactive Ingredients: Alcohol, Citric Acid, FD&C Red #40, Flavor,
Glycerin, Liquid Glucose, Purified Water, Saccharin Sodium, Sodium Benzoate.
CLINICAL PHARMACOLOGY
Codeine phosphate is an antitussive that is well recognized not only
because of its efficiency and rapidity of action but also because of its
relative safety in clinical use. Thus, irritating, nonproductive cough
is suppressed by codeine. The codeine content of PEDIACOF is reduced to
the proportion that is most suitable for children. When codeine is combined
with the expectorant potassium iodide, which tends to increase bronchial
secretion, coughing, although minimized, is more productive when it does
occur. The continuous fatiguing effect of useless coughing is thereby
avoided. Codeine is a narcotic analgesic and antitussive which resembles
morphine pharmacologically. Codeine is metabolized by the liver and excreted
chiefly in the urine, largely in inactive forms. A small fraction (10%)
of administered codeine is demethylated to form morphine, and both free
and conjugated morphine can be found in the urine after therapeutic doses
of codeine. When administered subcutaneously, 120 mg of codeine is approximately
equivalent to 10 mg of morphine. The abuse liability of codeine is generally
considered to be much lower than that of morphine.
The half-life of codeine in plasma is 2.5 to 3.0 hours.
Codeine has diverse additional actions. It depresses the respiratory center,
stimulates the vomiting center, depresses the cough reflex, constricts
the pupils, increases the tone of the gastrointestinal and genitourinary
tracts, and produces mild vasodilation.
Neo-Synephrine® , brand of phenylephrine hydrochloride, produces effective
decongestion of the mucous membranes of the respiratory tract via its
powerful postsynaptic  -receptor stimulant action. It has little effect
on cardiac
-receptor stimulant action. It has little effect
on cardiac  -receptors. Most of its effects are due to direct action on
receptors and only a small part is due to norepinephrine release. Central
stimulant activity is minimal.
-receptors. Most of its effects are due to direct action on
receptors and only a small part is due to norepinephrine release. Central
stimulant activity is minimal.
Chlorpheniramine maleate helps control allergic coughs and mucosal congestion.
The mild anticholinergic action of chlorpheniramine maleate may aid in
reducing rhinorrhea, and its mild sedative action may also be beneficial
to patients whose excessive coughing has caused them to lose sleep.
Clinical experience with PEDIACOF has shown it to be a dependable medication
for the relief of cough and the reduction of nasal congestion in children.
INDICATIONS AND USAGE
Coughs due to colds as well as coughs and congestive symptoms associated with upper respiratory tract infections such as tracheobronchitis or laryngobronchitis, croup, pharyngitis, allergic bronchitis, and infectious bronchitis, when accompanied by disturbing and fatiguing cough, have been treated successfully with PEDIACOF in children.
CONTRAINDICATIONS
PEDIACOF is contraindicated in patients who are hypersensitive to any of its ingredients. Due to the component phenylephrine, PEDIACOF is contraindicated in patients with ventricular tachycardia or severe hypertension.
WARNINGS
Respiratory Depression: Codeine produces dose-related respiratory depression
by acting directly on brain stem respiratory centers. Codeine also affects
centers that control respiratory rhythm and may produce irregular and
periodic breathing. If significant respiratory depression occurs, it may
be antagonized by the use of naloxone hydrochloride. (See OVERDOSAGE.)
Head Injury and Increased Intracranial Pressure: The respiratory depressant
effects of narcotics and their capacity to elevate cerebrospinal fluid
pressure may be markedly exaggerated in the presence of head injury, other
intracranial lesions, or a preexisting increase in intracranial pressure.
Furthermore, narcotics can produce adverse reactions which may obscure
the clinical course of patients with head injuries.
Acute Abdominal Conditions: The administration of narcotics may obscure
the diagnosis or clinical course of patients with acute abdominal conditions.
PRECAUTIONS
Caution should be exercised if PEDIACOF is administered to patients with cardiac disorders other than ventricular tachycardia, in which it is contraindicated; mild hypertension and hyperthyroidism.
Special Risk Patients:
Codeine should be used with caution in patients with impaired renal or
hepatic function, hypothyroidism, Addison’s disease, or urethral
stricture.
In asthma, the indiscriminate use of codeine may, due to its drying action
upon the mucosa of the respiratory tract, precipitate severe respiratory
insufficiency resulting from increased viscosity of the bronchial secretions
and suppression of the cough reflex. As with any narcotic analgesic agent,
the usual precautions should be observed and the possibility of respiratory
depression should be kept in mind.
Phenylephrine hydrochloride should be employed only with extreme caution
in patients with hyperthyroidism, bradycardia, partial heart block, and
myocardial disease.
Chlorpheniramine maleate should be used with considerable caution in patients
with narrow angle glaucoma, pyloroduodenal obstruction, and bladder neck
obstruction. Chlorpheniramine maleate has an atropine-like action and
therefore should be used with caution in patients with a history of bronchial
asthma, increased intraocular pressure, hyperthyroidism, cardiovascular
disease, and hypertension.
Drug Interactions: Patients receiving other narcotic analgesics, general
anesthetics, phenothiazines, tranquilizers, sedative-hypnotics, MAO inhibitors,
tricyclic antidepressants, or other CNS depressants (including alcohol)
concomitantly with codeine may exhibit an additive CNS depression. When
such combined therapy is contemplated, the dose of one or both agents
should be reduced.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No long-term animal
studies have been performed to evaluate the potential of PEDIACOF in these
areas.
Nonteratogenic Effects: Dependence has been reported in newborns whose
mothers received opiates regularly during pregnancy. Withdrawal signs
include irritability, excessive crying, tremors, hyperreflexia, fever,
vomiting, and diarrhea. Signs usually appear during the first few days
of life.
ADVERSE REACTIONS
The only significant untoward effects that have occurred are mild anorexia
and an occasional tendency to constipation. However, discontinuance of
PEDIACOF has seldom been required. Mild drowsiness occurs in some patients but, when cough
is relieved, the quieting effect of PEDIACOF is considered beneficial
in many instances. Because of its iodide content, PEDIACOF may cause elevation
of the protein-bound iodine. Adverse reactions to codeine include: Central
Nervous System: Sedation, drowsiness, mental clouding, dizziness, lethargy,
impairment of mental and physical performance, anxiety, convulsions, fear,
miosis, dysphoria, psychic dependence, mood changes, and respiratory depression.
Gastrointestinal System: Nausea, vomiting, increased pressure in the biliary
tract, and constipation. Cardiovascular System: Orthostatic hypotension,
fainting, and tachycardia. Genitourinary System: Ureteral spasm, spasm
of vesical sphincters and urinary retention have been reported. Other:
Flushing, sweating, pruritus, allergic reactions, and suppressed cough
reflex. Adverse reactions to phenylephrine hydrochloride include headache,
reflex bradycardia, excitability, restlessness, and, rarely, arrhythmias.
Adverse reactions to chlorpheniramine maleate include slight to moderate
drowsiness. Other possible side effects common to antihistamines in general
include: General: Urticaria, drug rash, anaphylactic shock, photosensitivity,
excessive perspiration, chills, dryness of mouth, nose, and throat. Cardiovascular
System: Hypotension, headache, palpitations, tachycardia, and extrasystoles.
Hematologic System: Hemolytic anemia, thrombocytopenia, and agranulocytosis.
Nervous System: Sedation, dizziness, disturbed coordination, fatigue,
confusion, restlessness, excitation, nervousness, tremor, irritability,
insomnia, euphoria, paresthesias, blurred vision, diplopia, vertigo, tinnitus,
acute labyrinthitis, hysteria, neuritis, and convulsions. Gastrointestinal
System: Epigastric distress, anorexia, nausea, vomiting, diarrhea, and
constipation. Genitourinary System: Urinary frequency, difficult urination,
urinary retention, and early menses. Respiratory System: Thickening of
bronchial secretions, tightness of chest and wheezing, and nasal stuffiness.
OVERDOSAGE
Codeine
Signs and Symptoms: Overdosage with codeine is characterized by respiratory
depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes
respiration, cyanosis), pinpoint pupils, extreme somnolence progressing
to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and
sometimes bradycardia and hypotension. In severe overdosage, particularly
by the intravenous route, apnea, circulatory collapse, cardiac arrest,
and death may occur.
Treatment: Primary attention should be given to the reestablishment of
adequate respiratory exchange through provision of a patent airway and
institution of assisted or controlled ventilation. Naloxone hydrochloride
is a specific and effective antagonist for respiratory depression which
may result from overdosage. If the desired degree of counteraction and
improvement in respiratory function is not obtained immediately following
IV administration, it may be repeated intravenously at 2 to 3 minute intervals.
Failure to obtain significant improvement after 2 or 3 doses suggests
that the condition may be due partly or completely to other disease processes
or nonopioid drugs. The usual initial pediatric dose is 0.01 mg/kg body
weight given IV, IM, or SC. If necessary, naloxone can be diluted with
Sterile Water for Injection, USP. Oxygen, intravenous fluids, vasopressors,
and other supportive measures should be employed as indicated.
Oral LD50 in the mouse is 693 mg/kg. Codeine is not dialyzable.
Phenylephrine hydrochloride
Signs and Symptoms: Overdosage may induce ventricular extrasystoles and
short paroxysms of ventricular tachycardia, a sensation of fullness in
the head, and tingling of the extremities.
Treatment: Should an excessive elevation of blood pressure occur, it may
be immediately relieved by an a-adrenergic blocking agent, e.g., phentolamine.
The oral LD50 in the rat: 350 mg/kg; mouse: 120 mg/kg.
Chlorpheniramine maleate
Signs and Symptoms: Antihistamine overdosage may vary from central nervous
system depression (sedation, apnea, and cardiovascular collapse) to stimulation
(insomnia, hallucinations, tremors or convulsions). Other signs and symptoms
may be dizziness, tinnitus, ataxia, blurred vision, and hypotension. Stimulation
and atropine-like signs and symptoms (dry mouth; fixed, dilated pupils;
flushing; hyperthermia, and gastrointestinal symptoms) are particularly
likely in children.
Treatment: Emergency treatment should be started immediately. Vomiting
should be induced, even if it has occurred spontaneously. Vomiting by
the administration of ipecac syrup is preferred. Vomiting should not be
induced in patients with impaired consciousness. The action of ipecac
is facilitated by physical activity and by the administration of eight
to twelve fluid ounces of water. If emesis does not occur within fifteen
minutes, the dose of ipecac should be repeated. Precautions against aspiration
must be taken, especially in infants and children. Following emesis, any
drug remaining in the stomach may be absorbed by activated charcoal administered
as a slurry with water. If vomiting is unsuccessful or contraindicated,
gastric lavage should be performed. Isotonic and one-half isotonic saline
are the lavage solutions of choice. Saline cathartics, such as milk of
magnesia, draw water into the bowel by osmosis and, therefore, may be
valuable for their action in rapid dilution of bowel content. After emergency
treatment the patient should continue to be medically monitored. Treatment
of the signs and symptoms of overdosage is symptomatic and supportive.
Stimulants (analeptic agents) should not be used. Vasopressors may be
used to treat hypotension. Short-acting barbiturates, diazepam, or paraldehyde
may be administered to control seizures. Hyperpyrexia, especially in children,
may require treatment with tepid water sponge baths or a hypothermic blanket.
Apnea is treated with ventilatory support.
DOSAGE AND ADMINISTRATION
PEDIACOF should be given in accordance with the needs and age of the patient. Frequency of administration may be adjusted as cough is brought under control. The following doses, to be given at 4 to 6 hour intervals, are suggested for patients under 12 years of age: from 6 months to 1 year, 1/4 teaspoon; from 1 to 3 years, ½ to 1 teaspoon; from 3 to 6 years, 1 to 2 teaspoons; and from 6 to 12 years, 2 teaspoons.
HOW SUPPLIED
Raspberry flavored syrup
Bottle of 16 fl oz (NDC 0024-1509-06)
Store at room temperature up to 25° C (77° F).
![]()
Manufactured for Sanofi-Synthelabo Inc.
New York, NY 10016
by Bayer Corporation
Myerstown, PA 17067
| Revised September 1999 | PSW-10D |