| NILANDRON® |
Prescribing Information |
|
| (nilutamide) Tablets |
DESCRIPTION |
CLINICAL PHARMACOLOGY
INDICATIONS AND USAGE |
CONTRAINDICATIONS
WARNINGS |
PRECAUTIONS
ADVERSE REACTIONS |
OVERDOSAGE
DOSAGE AND ADMINISTRATION |
HOW SUPPLIED
Rx only
DESCRIPTION
NILANDRON® tablets contain
nilutamide, a nonsteroidal, orally active antiandrogen having the chemical
name 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-2,4-imidazolidinedione
with the following structural formula:
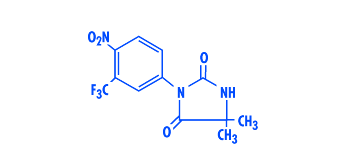
Nilutamide is a microcrystalline, white
to practically white powder with a molecular weight of 317.25. Its molecular formula is
C12H10F3N3O4.
It is freely soluble in ethyl acetate, acetone, chloroform, ethyl alcohol, dichloromethane,
and methanol. It is slightly soluble in water [< 0.1% W/V at 25°C (77°F)]. It melts between
153°C and 156°C (307.4°F and 312.8°F).
Each NILANDRON tablet contains 150 mg of nilutamide. Other ingredients in NILANDRON tablets
are corn starch, lactose, povidone, docusate sodium, magnesium stearate, and talc.
BACK TO TOP
CLINICAL PHARMACOLOGY
Mechanism of Action
Prostate cancer is known to be androgen sensitive and responds
to androgen ablation. In animal studies, nilutamide has demonstrated
antiandrogenic activity without other hormonal (estrogen, progesterone,
mineralocorticoid, and glucocorticoid) effects. In vitro, nilutamide
blocks the effects of testosterone at the androgen receptor level. In
vivo, nilutamide interacts with the androgen receptor and prevents the
normal androgenic response.
Pharmacokinetics
Absorption:
Analysis of blood, urine, and feces samples following
a single oral 150-mg dose of [14C]-nilutamide in patients
with metastatic prostate cancer showed that the drug is rapidly and
completely absorbed and that it yields high and persistent plasma concentrations.
Distribution:
After absorption of the drug, there is a detectable distribution phase. There is moderate
binding of the drug to plasma proteins and low binding to erythrocytes. The binding is
nonsaturable except in the case of alpha-1-glycoprotein, which makes a minor contribution to the
total concentration of proteins in the plasma. The results of binding studies do not indicate any effects that
would cause nonlinear pharmacokinetics.
Metabolism:
The results of a human metabolism
study using 14C-radiolabelled tablets show that nilutamide
is extensively metabolized and less than 2% of the drug is excreted
unchanged in urine after 5 days. Five metabolites have been isolated
from human urine. Two metabolites display an asymmetric center, due
to oxidation of a methyl group, resulting in the formation of D- and
L-isomers. One of the metabolites was shown, in vitro, to possess 25
to 50% of the pharmacological activity of the parent drug, and the D-isomer
of the active metabolite showed equal or greater potency compared to
the L-isomer. However, the pharmacokinetics and the pharmacodynamics
of the metabolites have not been fully investigated.
Elimination:
The majority (62%)
of orally administered [14C]-nilutamide is eliminated in
the urine during the first 120 hours after a single 150-mg dose. Fecal
elimination is negligible, ranging from 1.4% to 7% of the dose after
4 to 5 days. Excretion of radioactivity in urine likely continues beyond
5 days. The mean elimination half-life of nilutamide determined in studies
in which subjects received a single dose of 100-300 mg ranged from 38.0
to 59.1 hours with most values between 41 and 49 hours. The elimination
of at least one metabolite is generally longer than that of unchanged
nilutamide (59-126 hours). During multiple dosing of 150 mg nilutamide
(given as 3 x 50 mg) twice a day, steady state was reached within 2
to 4 weeks for most patients, and mean steady state AUC0-12
was 110% higher than the AUC0- obtained from the first 150 mg dose. These data and in vitro
metabolism data suggest that, upon multiple dosing, metabolic enzyme
inhibition may occur for this drug.
obtained from the first 150 mg dose. These data and in vitro
metabolism data suggest that, upon multiple dosing, metabolic enzyme
inhibition may occur for this drug.
Clinical Studies
Nilutamide through its antiandrogenic activity can complement surgical
castration, which suppresses only testicular androgens. The effects
of the combined therapy were studied in patients with previously untreated
metastatic prostate cancer.
In a double-blind, randomized, multicenter study that enrolled 457 patients (225 treated with
orchiectomy and NILANDRON, 232 treated with orchiectomy and placebo), the NILANDRON group showed
a statistically significant benefit in time to progression and time to death. The results are summarized below.
|
NILANDRON |
PLACEBO |
| Median Survival (months) |
27.3 |
23.6 |
| Progression-Free Survival (months) |
21.1 |
14.9 |
| Complete or Partial Regression |
41% |
24% |
| Improvement in Bone Pain |
54% |
37% |
BACK TO TOP
INDICATIONS AND USAGE
Metastatic Prostate Cancer
NILANDRON tablets are indicated for use in combination with surgical
castration for the treatment of metastatic prostate cancer (Stage D2).
For maximum benefit, NILANDRON treatment must
begin on the same day as or on the day after surgical castration.
BACK TO TOP
CONTRAINDICATIONS
NILANDRON tablets are contraindicated:
in patients with severe hepatic impairment
(baseline hepatic enzymes should be evaluated prior to treatment)
-
in patients with severe respiratory
insufficiency
-
in patients with hypersensitivity
to nilutamide or any component of this preparation.
BACK TO TOP
WARNINGS
|
Interstitial Pneumonitis
Interstitial pneumonitis has been reported in 2% of patients
in controlled clinical trials in patients exposed to nilutamide.
A small study in Japanese subjects showed that 8 of 47 patients
(17%) developed interstitial pneumonitis. Reports of interstitial
changes including pulmonary fibrosis that led to hospitalization
and death have been reported rarely post-marketing. Symptoms
included exertional dyspnea, cough, chest pain, and fever. X-rays
showed interstitial or alveolo-interstitial changes, and pulmonary
function tests revealed a restrictive pattern with decreased
DLco. Most cases occurred within the first 3 months of treatment
with NILANDRON, and most reversed with discontinuation of therapy.
A routine chest X-ray should be performed prior to initiating
treatment with NILANDRON. Baseline pulmonary function tests
may be considered. Patients should be instructed to report any
new or worsening shortness of breath that they experience while
on NILANDRON. If symptoms occur, NILANDRON should be immediately
discontinued until it can be determined if the symptoms are
drug related.
|
Hepatitis
Rare cases of death or hospitalization due to severe
liver injury have been reported post-marketing in association with the
use of NILANDRON. Hepatotoxicity in these reports generally occurred
within the first 3 to 4 months of treatment. Hepatitis or marked increases
in liver enzymes leading to drug discontinuation occurred in 1% of NILANDRON
patients in controlled clinical trials.
Serum transaminase levels should be measured prior to starting treatment with NILANDRON,
at regular intervals for the first 4 months of treatment, and periodically thereafter.
Liver function tests should also be obtained at the first sign or symptom suggestive of
liver dysfunction, e.g. nausea, vomiting, abdominal pain, fatigue, anorexia, “flu-like”
symptoms, dark urine, jaundice, or right upper quadrant tenderness. If at any
time, a patient has jaundice, or their ALT rises above 2 times the upper limit of normal,
NILANDRON should be immediately discontinued with close followup of liver function tests
until resolution.
Use in Women
NILANDRON has no indication for women, and should not be used in this population, particularly for
non-serious or non-life threatening conditions.
Other
Foreign postmarketing surveillance has revealed isolated cases of aplastic anemia in which
a causal relationship with NILANDRON could not be ascertained.
BACK TO TOP
PRECAUTIONS
General
Antiandrogen Withdrawal Syndrome
Patients whose disease progresses, while being treated with an antiandrogen may experience
clinical improvement with discontinuation of the antiandrogen.
Information for Patients
Patients should be informed that NILANDRON tablets should be started on the day of, or on the day
after, surgical castration. They should also be informed that they should not interrupt their
dosing of NILANDRON or stop taking this medication without consulting their physician.
Because of the possibility of interstitial pneumonitis, patients should also be told to report
immediately any dyspnea or aggravation of pre-existing dyspnea.
Because of the possibility of hepatitis, patients should be told to consult with their physician
should nausea, vomiting, abdominal pain, or jaundice occur.
Because of the possibility of an intolerance to alcohol (facial flushes, malaise, hypotension)
following ingestion of NILANDRON, it is recommended that intake of alcoholic beverages be avoided
by patients who experience this reaction. This effect has been reported in about 5% of patients
treated with NILANDRON.
In clinical trials, 13% to 57% of patients receiving NILANDRON reported a delay in adaptation to
dark, ranging from seconds to a few minutes, when passing from a lighted area to a dark area. This
effect sometimes does not abate as drug treatment is continued. Patients who experience this effect
should be cautioned about driving at night or through tunnels. This effect can be alleviated by the
wearing of tinted glasses.
Drug Interactions
In vitro, nilutamide has been shown to inhibit the activity of liver cytochrome
P-450 isoenzymes and, therefore, may reduce the metabolism of compounds
requiring these systems.
Consequently, drugs with a low therapeutic margin, such as vitamin K antagonists, phenytoin,
and theophylline, could have a delayed elimination and increases in their serum half-life leading
to a toxic level. The dosage of these drugs or others with a similar metabolism may need to be
modified if they are administered concomitantly with nilutamide. For example, when vitamin K
antagonists are administered concomitantly with nilutamide, prothrombin time should be carefully
monitored and, if necessary, the dosage of vitamin K antagonists should be reduced.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Administration of nilutamide to rats for 18 months at doses of 0, 5, 15, or 45 mg/kg/day
produced benign Leydig cell tumors in 35% of the high-dose male rats (AUC exposures
in high-dose rats were approximately 1 - 2 times human AUC exposures
with therapeutic doses). The increased incidence of Leydig cell tumors
is secondary to elevated luteinizing hormone (LH) concentrations resulting
from loss of feedback inhibition at the pituitary. Elevated LH and testosterone
concentrations are not observed in castrated men receiving NILANDRON.
Nilutamide had no effect on the incidence, size, or time of onset of
any spontaneous tumor in rats.
Nilutamide displayed no mutagenic effects in a variety of in vitro and in vivo tests (Ames test, mouse micronucleus
test, and two chromosomal aberration tests).
In reproduction studies in rats, nilutamide had no effect on the reproductive function of males and females,
and no lethal, teratogenic, or growth-suppressive effects on fetuses were found. The maximal dose at which
nilutamide did not affect reproductive function in either sex or have an effect on fetuses was estimated to
be 45 mg/kg orally (AUC exposures in rats approximately 1-2 times human therapeutic AUC exposures).
Pregnancy
Pregnancy Category C; Animal reproduction studies have not been conducted with nilutamide.
It is also not known whether nilutamide can cause fetal harm when administered
to a pregnant woman or can affect reproductive capacity. Nilutamide
should be given to a pregnant woman only if clearly needed.
Pediatric Use
Safety and effectiveness in pediatric patients have not been determined.
Animal Pharmacology and Toxicology
Administration of NILANDRON to beagle dogs resulted in drug-related deaths at dose
levels that produce AUC exposures in dogs much lower than the AUC exposures
of men receiving the therapeutic doses of 150 and 300 mg/day. Nilutamide-induced
toxicity in dogs was cumulative with progressively lower doses producing
death when given for longer durations. Nilutamide given to dogs at 60
mg/kg/day (1-2 times human AUC exposure) for 1 month produced 100% mortality.
Administration of 20 and 30 mg/kg/day nilutamide (1/2-1 times human
AUC exposure) for 6 months resulted in 20% and 70% mortality in treated
dogs. Administration to dogs of 3, 6, and 12 mg/kg/day nilutamide (1/10-1/2
human AUC exposure) for 1 year resulted in 8%, 33%, and 50% mortality,
respectively. A “no-effect level” for nilutamide-induced
mortality in dogs was not identified. Pathology data from the one-year
oral toxicity study suggest that the deaths in dogs were secondary to
liver toxicity. Marked-to-massive hepatocellular swelling and vacuolization
were observed in affected dogs. Liver toxicity in dogs was not consistently
associated with elevations of liver enzymes.
Administration of nilutamide to rats at a dose level of 45 mg/kg/day (AUC exposure in rats
1-2 times human therapeutic AUC exposures) for 18 months increased the incidence of lung
pathology (granulomatous inflammation and chronic alveolitis).
The hepatic and pulmonary adverse effects
observed in nilutamide-treated animals and men are similar to effects
observed with another nitroaromatic compound, nitrofurantoin. Nilutamide
and nitrofurantoin are both metabolized in vitro to nitroanion free-radicals
by microsomal NADPH-cytochrome P450 reductase in the lungs and liver
of rats and humans.
BACK TO TOP
ADVERSE REACTIONS
The following adverse experiences were reported
during a multicenter clinical trial comparing NILANDRON + surgical castration
versus placebo + surgical castration. The most frequently reported (greater
than 5%) adverse experiences during treatment with NILANDRON tablets
in combination with surgical castration are listed below. For comparison,
adverse experiences seen with surgical castration and placebo are also
listed.
|
Adverse Experience
|
NILANDRON
+
surgical castration
(N=225)
% All
|
Placebo
+
surgical castration
(N=232)
% All
|
|
Cardiovascular System
Hypertension
Digestive System
Nausea
Constipation
Endocrine System
Hot flushes
Metabolic and Nutritional
System
Increased AST
Increased ALT
Nervous System
Dizziness
Respiratory System
Dyspnea
Special Senses
Impaired adaptation
to dark
Abnormal vision
Urogenital System
Urinary tract infection
|
5.3
9.8
7.1
28.4
8.0
7.6
7.1
6.2
12.9
6.7
8.0
|
2.6
6.0
3.9
22.4
3.9
4.3
3.4
7.3
1.3
1.7
9.1
|
The overall incidence of adverse experiences was
86% (194/225) for the NILANDRON group and 81% (188/232) for the placebo group.
The following adverse experiences were reported
during a multicenter clinical trial comparing NILANDRON + leuprolide
versus placebo + leuprolide. The most frequently reported (greater than
5%) adverse experiences during treatment with NILANDRON tablets in combination
with leuprolide are listed below. For comparison, adverse experiences
seen with leuprolide and placebo are also listed.
|
Adverse Experience
|
NILANDRON
+
leuprolide
(N=209)
% All
|
Placebo
+
leuprolide
(N=202)
% All
|
|
Body as a Whole
Pain
Headache
Asthenia
Back pain
Abdominal pain
Chest pain
Flu syndrome
Fever
Cardiovascular System
Hypertension
Digestive System
Nausea
Constipation
Anorexia
Dyspepsia
Vomiting
Endocrine System
Hot flushes
Impotence
Libido decreased
Hemic and Lymphatic
System
Anemia
Metabolic and
Nutritional System
Increased AST
Peripheral edema
Increased ALT
Musculoskeletal
System
Bone Pain
Nervous System
Insomnia
Dizziness
Depression
Hypesthesia
Respiratory System
Dyspnea
Upper respiratory
infection
Pneumonia
Skin and Appendages
Sweating
Body hair loss
Dry skin
Rash
Special Senses
Impaired adaptation
to dark
Chromatopsia
Impaired adaptation
to light
Abnormal vision
Urogenital System
Testicular atrophy
Gynecomastia
Urinary tract infection
Hematuria
Urinary tract disorder
Nocturia
|
26.8
13.9
19.1
11.5
10.0
7.2
7.2
5.3
9.1
23.9
19.6
11.0
6.7
5.7
66.5
11.0
11.0
7.2
12.9
12.4
9.1
6.2
16.3
10.0
8.6
5.3
10.5
8.1
5.3
6.2
5.7
5.3
5.3
56.9
8.6
7.7
6.2
16.3
10.5
8.6
8.1
7.2
6.7
|
27.7
10.4
20.8
16.8
5.4
4.5
3.0
6.4
9.9
8.4
16.8
6.4
4.5
4.0
59.4
12.9
4.5
6.4
13.9
17.3
8.9
5.0
15.8
11.4
7.4
2.0
7.4
10.9
3.5
3.0
0.5
2.5
4.0
5.4
0.0
1.0
4.5
12.4
11.9
21.3
7.9
10.4
6.4
|
The overall incidence of adverse experiences is 99.5% (208/209) for the
NILANDRON group and 98.5% (199/202) for the placebo group.
Some frequently occurring adverse experiences, for example hot flushes, impotence, and decreased libido, are known
to be associated with low serum androgen levels and known to occur with medical or surgical castration alone. Notable
was the higher incidence of visual disturbances (variously described as impaired adaptation to darkness, abnormal
vision, and colored vision), which led to treatment discontinuation in 1% to 2% of patients.
Interstitial pneumonitis occurred in one (<1%) patient receiving NILANDRON in combination with surgical
castration and in seven patients (3%) receiving NILANDRON in combination with leuprolide and one patient
receiving placebo in combination with leuprolide. Overall, it has been reported in 2% of patients receiving
NILANDRON. This included a report of interstitial pneumonitis in 8 of 47 patients (17%) in a small study
performed in Japan.
In addition, the following adverse experiences were reported in 2 to 5% of patients treated with NILANDRON
in combination with leuprolide or orchiectomy.
Body as a Whole: Malaise (2%).
Cardiovascular System: Angina (2%), heart failure (3%), syncope (2%).
Digestive System: Diarrhea (2%), gastrointestinal disorder (2%), gastrointestinal hemorrhage (2%), melena (2%).
Metabolic and Nutritional System: Alcohol intolerance (5%), edema (2%), weight loss (2%).
Musculoskeletal System: Arthritis (2%).
Nervous System: Dry mouth (2%), nervousness (2%), paresthesia (3%).
Respiratory System: Cough increased (2%), interstitial lung disease (2%), lung disorder (4%), rhinitis (2%).
Skin and Appendages: Pruritus (2%).
Special Senses: Cataract (2%), photophobia (2%).
Laboratory Values: Haptoglobin increased (2%), leukopenia (3%), alkaline phosphatase increased (3%), BUN increased
(2%), creatinine increased (2%), hyperglycemia (4%).
BACK TO TOP
OVERDOSAGE
One case of massive overdosage has been published. A 79-year-old man
attempted suicide by ingesting 13 g of nilutamide (i.e., 43 times the maximum recommended dose). Despite immediate
gastric lavage and oral administration of activated charcoal, plasma nilutamide levels peaked at 6 times the normal
range 2 hours after ingestion. There were no clinical signs or symptoms or changes in parameters such
as transaminases or chest X-ray. Maintenance treatment (150 mg/day) was resumed 30 days later.
In repeated-dose tolerance studies, doses of 600 mg/day and 900 mg/day were administered to 9 and 4 patients,
respectively. The ingestion of these doses was associated with gastrointestinal disorders, including nausea
and vomiting, malaise, headache, and dizziness. In addition, a transient elevation in hepatic enzyme levels
was noted in one patient.
Since nilutamide is protein bound, dialysis may not be useful as treatment for overdose. As in the
management of overdosage with any drug, it should be borne in mind that multiple agents may have
been taken. If vomiting does not occur spontaneously, it should be induced
if the patient is alert. General supportive care, including frequent
monitoring of the vital signs and close observation of the patient,
is indicated.
BACK TO TOP
DOSAGE AND ADMINISTRATION
The recommended dosage is 300 mg once a day for
30 days, followed thereafter by 150 mg once a day. NILANDRON tablets
can be taken with or without food.
BACK TO TOP
HOW SUPPLIED
NILANDRON 150 mg tablets are supplied
in boxes of 30 tablets. Each box contains 3 child-resistant, PVC, aluminum
foil-backed blisters of 10 tablets (NDC 0088-1111-14). Each white, biconvex,
cylindrical (10 mm in diameter) tablet has a triangular logo on one
side and an internal reference number (168D) on the other.
Store at 25°C (77°F); excursions permitted between 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Protect from light.
Revised June 2006
sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
Country of Origin: France
© 2006 sanofi-aventis U.S. LLC
DESCRIPTION |
CLINICAL PHARMACOLOGY
INDICATIONS AND USAGE |
CONTRAINDICATIONS
WARNINGS |
PRECAUTIONS
ADVERSE REACTIONS |
OVERDOSAGE
DOSAGE AND ADMINISTRATION |
HOW SUPPLIED
View Acrobat PDF
|
